All published articles of this journal are available on ScienceDirect.
Percutaneous Endoscopic Spine Surgery for Extruded Lumbar Disc Herniation
Abstract
The conventional open discectomy is the gold standard for treating extruded lumbar disc herniation, especially in highly migrated lumbar disc herniation. Endoscopic spine surgery is known to be very challenging and technically demanding, in particular for highly migrated disc herniation. However, several studies have reported numerous effective techniques with results approximatively equal to conventional open surgeries or mini-open surgery. In the last few years, an increased number of endoscopic spine surgical techniques have been proposed in order to overcome various issues encountered in traditional endoscopic spine surgery. Nevertheless, surgical approach selection for treating extruded lumbar disc herniation is based on aspects such as anatomical structures, availability of surgical instruments, surgeon’s experience, and the disc herniation location. Advances in endoscopic visualization and instrumentation, as well as an increased demand for minimally invasive procedures, have led to the popularity of Percutaneous Endoscopic Lumbar Discectomy (PELD). PELD is a recent and advanced technique among other minimally invasive spine surgeries (MIS). It includes various kinds of surgical techniques to treat lumbar disc herniation and aims to offer a safe, less invasive surgical procedure for lumbar disc space decompression and removal of nucleus pulposus.
1. INTRODUCTION
Degenerative lumbar disc disease is a common condition affecting approximately 90% of adults population during their lifetime [1, 2]. Disc herniation is one of the stages of the degenerative cascade. Symptoms like back pain and leg pain are usually related to Lumbar Disk Herniation (LDH). The surgical treatment for this condition has developed from traditional open spine surgeries to minimal access spine surgeries including endoscopic spine surgeries. In 1964, Smith introduced the first minimally invasive spine surgery known as percutaneous lumbar chemonucleolysis [3]. A few years later, minimal access spine surgery for lumbar disc herniation was first reported by Kambin and colleague and Hijikata in 1975 [4, 5].
Percutaneous Endoscopic Lumbar Discectomy (PELD) is a new technique among other Minimally Invasive Spine surgeries (MIS). The basis for percutaneous lumbar disc procedures originated from Posterolateral percutaneous biopsy techniques of the lumbar vertebrae. These techniques were performed with the use of a Craig needle originally used to perform the percutaneous biopsy of neoplastic conditions [6, 7]. There are several other nonvisualized percutaneous techniques that often get confused and classified as posterolateral endoscopic lumbar discectomy. These include percutaneous laser discectomy, Automated Percutaneous Lumbar Discectomy (APLD), and percutaneous discectomy with the Dekompressor.(a device introduced in 2002 and used for tissue removal through access to the disc nucleus) [8] All the aforementioned techniques are fluoroscopically guided nonvisualized procedures that access the disc via the same posterolateral approach as endoscopic lumbar surgery. Numerous studies reported decreases in intradiscal pressures of 50% or greater [9, 10]. The results of these types of indirect decompressive procedures have been comparable with initial favorable reports. However, later studies have shown varying degrees of success. Compared to these procedures, PELD has enhanced the capabilities of foraminal endoscopic discectomy to deliver surgical results comparable to traditional open spine surgeries in the treatment of common lumbar disc herniations. The advancement of this new technique emphasized a closer placement of the cannula to the epidural space and the base of the targeted disc herniation. This facilitated surgeons to target extruded herniations in addition to contained herniations. Nevertheless, its application in highly migrated Lumbar disc herniation remains a challenging and demanding task. This review is focused on the most common percutaneous endoscopic surgical approaches for a lumbar herniated disc with migration.
2. DISC HERNIATION CLASSIFICATION
Clinicians and radiologists need to be familiar with standard terms for normal and pathologic conditions of lumbar discs; clear understandings of these terms are essential for an accurate clinical diagnostic and a reasonable therapeutic decision-making [11].
Disc herniation is the manifestation of a lumbar degenerative disease. The term “herniated disc” refers to the displacement of disc material beyond the normal limit of intervertebral disc space. The herniation may contain a nucleus, cartilage, fragmented apophyseal bone, or fragmented annular tissue. [9, 8]
Various systems of classification have been suggested for lumbar disc herniation, however, none is comprehensive or perfect, and are mostly used as tools to describe the herniation. Based on previous studies, herniated disc can be divided into protrusion and extrusion [11] (Fig. 1).
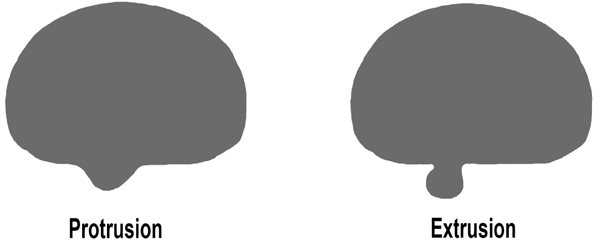
2.1. Protruded Disc Herniation
Protruded disc refers to focal or localized abnormalities of the disc in which the displaced disc material has not extended beyond the limit of annulus fibrosus; i.e. the displaced disc material is continuous with the remaining material within the disc space and is less than 25% of the disc circumference. [9, 8]
2.2. Extruded Disc Herniation
An extruded disc is defined as disc material that has extended beyond annulus fibrous but, remains partially in continuity with the disc of origin. Extruded disc material that has no continuity with the disc of origin may be characterized as sequestrated [11]. This is the typical “free fragment” Sequestrated disc, a subtype of “extruded disc”. Extruded disc material that is displaced away from the location of extrusion, regardless of continuity with the disc, may be called “migrated”. This may be helpful for imaging studies interpretation because in some cases, it is impossible to be certain if the continuity still exists from image examination alone. [9, 8]
Other authors divided herniation into two types: contained and uncontained.
Contained herniation refers to herniated disc materials that have not passed beyond the limit of the posterior longitudinal ligament or the outer layer of the annulus, whereas uncontained herniation refers to those that have crossed this margin. Displaced disc fragments are sometimes characterized as “free”. A “free fragment” is synonymous with a “sequestrated fragment”, but not with “uncontained”. A disc fragment should be considered “free” or “sequestrated” only if there is no remaining continuity of the disc material between it and the disc of origin [8, 10]. A disc can be “uncontained”, with the loss of integrity of the posterior longitudinal ligament and the outer annulus, but still, have continuity between the herniated/displaced disc material and the disc of origin. The technical limitations of CT and MRI imaging are difficult to distinguish between contained and uncontained herniation [11].
3. DISC HERNIATION LOCATION
Disc herniations can also be described by their location and are divided into 5 anatomic zones (Fig. 2).
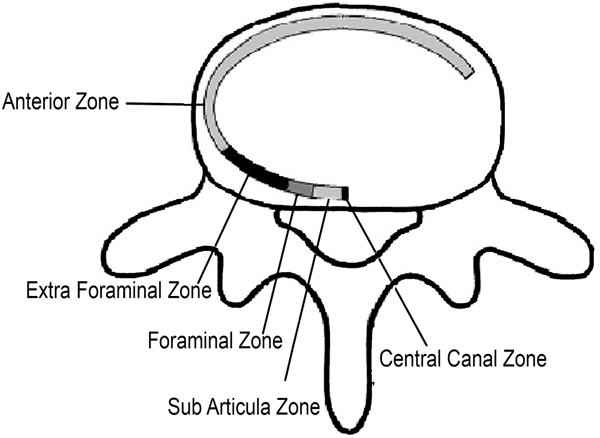
3.1. Central
The disc herniation is located within the boundaries of the cauda equina dural sac.
3.2. Subarticula
(r): It is also known as a lateral recess or paracentral, it is bordered by the lateral aspect of the dural sac and the medial aspect of the pedicle and neural foramen. In this zone, the nerve root moves downward toward its respective foramen.
3.3. Foraminal
The disc herniation is located between the medial and lateral borders of the pedicle.
3.4. Extraforaminal and Anterior
Also known as lateral or far lateral, refers to herniations located beyond the lateral border of the pedicle but within the far-lateral or extraforaminal zone. In some case, herniation can reach the anterior region. Herniations in the extraforaminal region usually affect exiting nerve roots.
Lateral disc herniation generally irritates and compresses the ganglia of the dorsal root causing radiculopathy with severe pain [11, 12]. Most foraminal disc herniations are accompanied by radiculopathy, usually referred to as acute radicular syndromes. This radiculopathy comes from the compression of the longitudinal emerging root in the extra canal portion of the medullary canal. These anatomic considerations can explain the high prevalence of radiculopathy in subarticular and foraminal regions in our population. Foraminal or extraforaminal Lumbar disc herniation that extends beyond the foraminal zone accounts for 7–12% of all Lumbar Disc Herniation cases (LDH) [13, 14]. A nearly equal incidence of LDH occurring at the L4/5 and L3/4 level has been reported [16]. Moreover, patients with foraminal or extraforaminal lumbar disc herniation usually have an age range from 50–78 years old [16].
In the sagittal plane, the location may be defined as “discal”, “suprapedicular”, “pedicular”, and “Infrapedicular”. [12] The pedicle and disc are used as reference points (Fig. 3).
Disc herniation may extend to:
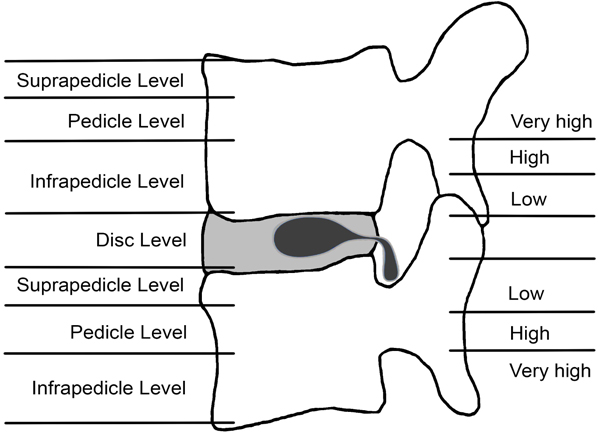
3.5. Disc Level
Confined between the vertebral endplates.
3.6. Suprapedicular
Between superior endplate and the superior border of the pedicle.
3.7. Pedicular
At the level of the pedicle.
3.8. Infrapedicular
Below lower margin of the pedicle to the inferior endplate.
4. PELD FOR MIGRATED DISC HERNIATION
The most popular surgical approaches for PELD are transforaminal and interlaminar approaches. Various studies reported that PELD is effective and offers numerous advantages such as direct visualization of the pathology, reduced soft tissue trauma, reduced blood loss, quicker recovery and preservation of the adjacent anatomy over open discectomy including microdiscectomy [15, 16]. PELD is performed with the patient under local anesthesia in the prone position.
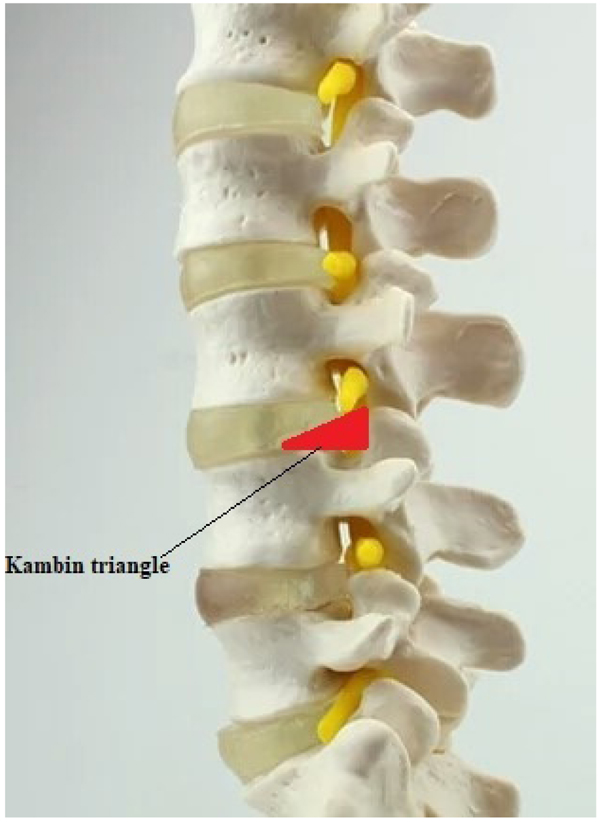
In the history of minimally invasive spine surgery, Kambin was the first to describe and illustrate the boundaries of a safe working zone for the posterolateral approach known as Kambin's triangle in 1990 (Fig. 4). He described the radiological positioning of the needle in anteroposterior and lateral views. This helped previous percutaneous modalities which were blindly conducted and have opened a way to new techniques. [7, 17, 18].
5. PELD ADVANTAGES AND DISADVANTAGES
5.1. Advantages
- Minimizes the risks of bleeding, infection, neural and nonpathological disc nucleus injury ;
- Less post-operative pain;
- Short recovery period and fast return to active lifestyle;
- risks of nerve injury or thrombosis are significantly reduced; less cardiac cycle stress;
- Direct access to the migrated or sequestered herniated disc;
- Immediate pain relief after the surgical procedure in 90% of cases;
- Reduced length of hospital stay.
6. MIGRATED DISC HERNIATION
The term “migrated” disc or fragment refers to the displacement of most of the disc material away from the annulus opening through which the material has extruded. Some migrated fragments will be sequestrated, but the term “migrated” refers only to position and not to continuity [11]. The classification and grading system of migrated disc herniation was first proposed by Lee and colleague [22], then later modified by Kim et al. [23] based on recent studies migrated disc herniation can be divided into three types: low- grade, high- grade, and very high grade [19, 20, 21]. Figs. (3 and 5) A migrated disc herniation is defined as low -grade when the extent of migration is less than the posterior marginal height, whereas a high-grade migration refers to when the extent of the disc migration is more than the posterior marginal disc height. Kim et al [23], defined as very high- grade when the disc migration extends beyond the inferior margin of the pedicle in either cranial or caudal direction.
7. CRANIAL OR CAUDAL MIGRATION
Regarding disc herniation migration, the mechanism of the migrational direction is still poorly understood. Recent studies reported that the incidence rate of cranial and caudal migration of herniated lumbar disc material varies between 40% and 78% or 40% and 50% [22-26]. Schellinger et al. [27] suggested that the Herniated Nucleus Pulposus (HNP) appears to have an equal chance of moving cranially or caudally because the structure of the Anterior Epidural Space (AES) is the same at both superior and inferior halves of the vertebral body. Whereas Fries et al. [26] assumed that a higher rate of cranial migration of herniated disc material may occur because of greater space available to contain herniated nucleus pulposus in the anterior epidural. M.Daghighi et al. [14] Found that there is a higher incidence of cranial lumbar migration in older patients. They also supported the idea that both age and level of herniation are independently associated with the direction of migrated disc materials. This is in accordance with other studies which reported that the age and anatomic structural changes during aging; may justify the connection between age and the location of migrated disc materials [28-30]. Age-related changes in the AES along with other senile degenerations may contribute to the higher incidence of cranial migration in elderly [14].
Anatomical structures may be the cause for caudal migration i.e. the foramina becomes enlarged in the caudal portions of the vertebral column, the number of attachment points at the posterior wall of the vertebral bodies decreases caudally this may explain the tendency of caudal migration of the herniated disc materials in the lower segments of the lumbar spine [14].
It is essential to take into account that herniated disc migration may be affected by other factors as well such as the degree of lordosis, previous asymptomatic disc herniation, anatomical variations, facet atropism, distribution of intervertebral stress, and preexisting status of the trunk muscles [27, 30, 31]. Many of these factors are interconnected [32].
8. POSTERIOR EPIDURAL MIGRATION
Disc fragments are known to migrate to cranial or caudal directions or even laterally in the anterior epidural space [27, 33, 34]. In 1973 Lombardi [35], reported the first case of Posterior epidural lumbar disc fragments migration. Posterior epidural lumbar migration of an extruded disc fragment is a very rare clinical case with an unclear pathogenesis. Recent studies showed a higher occurrence of posterior epidural lumbar in men than women and it usually involves middle age individuals. It is more frequent at the L3-L4 level [36, 37] where it may lead to cauda equina compression. Posterior epidural migration is often misdiagnosed or confused with other posterior epidural lesions [33, 36, 37] such as hematomas, cysts, abscesses, tumors. The radiological diagnosis for posterior epidural migrated lumbar disc fragments is quite challenging due to the unusual location and in some cases, a potential stimulation of tumors or other epidural pathologies due to contrast enhancement and mass effects. Magnetic Resonance (MR) can help differentiate this pathology from other lesions. Although this condition has been extensively mentioned, none have reported comprehensive studies for this pathology [41]. In a previously published study, the authors assumed that the mechanism leading to epidural space narrowing may be the cause of posterior migration of sequestered disc fragment [41]. The proximity of the annulus fibrous tear to the pedicle and an acute strong pressure may push the disc fragment to the dorsal side of the dural sac. Concerning an endoscopic surgical treatment for this condition, only a few numbers of studies have reported a successful outcome. [36-38] Therefore, further research is needed.
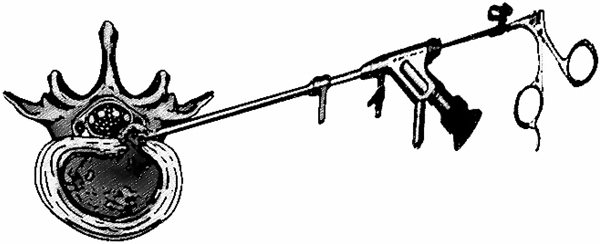
9. THE YESS SYSTEM “INSIDE-OUT” TECHNIQUE
Anthony Yeung developed the first working channel in 1997 [39, 40]. This endoscopic new system offered a significant visual improvement and a complementary instrument system with specialized beveled and slotted cannulas with the open end directed toward the dorsal foramen. It is referred to as the YESS (Yeung Endoscopic Spine Surgery) technique also known as “inside-out” technique (Fig. 6). Yeung’s technique was based on the principle of identification and treatment of pain generators located into the foramen and the disc. It consists of releasing exiting and traversing roots by fragmentectomy, visualization and clearance of annular tear by ablation and irrigation. [15, 9, 41]
The aforementioned technique is indicated for most lumbar disc herniation but, its application for extruded lumbar disc herniation is limited due to several reasons such as poor visualization, anatomic barriers, and incomplete disc fragments removal. Lee et al. [22] has suggested a conventional inside-out technique called the “Half-and-Half” technique to remove the near- migrated disc. This technique consists of positioning the window of beveled working sheath across the disc space to the epidural space. Other studies also reported that the Low-grade migrated disc can be successfully removed with the “inside-out” technique without difficulty [19, 20]. However, endoscopic discectomy for highly migrated lumbar disc herniation has been known to be very challenging even for an experienced spine surgeon.
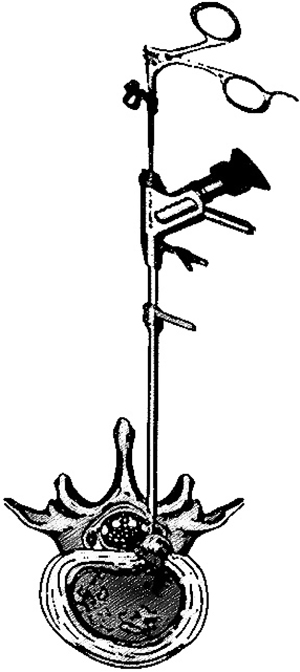
10. TESSYS (Transforaminal Endoscopic Surgical System)
TESSYS (Transforaminal Endoscopic Surgical System) also knows as “outside-in” technique (Fig.7) is a development of the existing transforaminal access technique (Yess technique) proposed by Dr. Thomas Hoogland. This method consists of cutting the undersurface of the facet to get into the epidural space [4, 42]. Various studies have reported this method to be effective for extruded lumbar disc herniation. However, its application in migrated or sequestered disc herniation has encountered various insufficiencies and limitation which led to surgical failure with approximatively about 8.5 -15.7% reoperation rate [43, 44]. Various studies reported that anatomical structures, poor visualization, and the lack of a standard surgical guideline for an inexperienced surgeon might be the causes of surgical failure [47]. To overcome these limitations, several approaches and techniques were developed in order to decrease the failure rate and improve surgical efficiency. TESSYS has fewer postoperative complications than conservative surgery, with a mean complication rate of 2.8% [45, 46]
11. THE OUTSIDE-IN TECHNIQUE WITH FORAMINOPLASTY
Foraminoplasty is an additional technique which can be combined with the conventional PELD, using instruments called reamers (endoscopic drills or trephine). Many authors have reported this strategy to be effective and beneficial for resolving PELD limitation [43, 47-50]. It helps surgeons have adequate working space for an easy management of the pathologic lesion in the epidural space but also have an access to the disc fragments in the hidden zone far from disc space. The term “Hidden zone” was introduced by Macnab in 1971 to describe the lateral zone of the lumbar spine and point out difficulties related to exposing the lesion [51, 54].
The conventional foraminoplasty consists of removing the lateral edge of the ligamentum flavum and undercutting nonarticular part of the superior facet joint. In High- grade downward migrated disc, the use of a rigid endoscope in the conventional technique cannot help visualizing the entire fragment. Hoogland and Schuber et al. [50] failed to completely visualize the fragment below the mid-pedicle of the low vertebra after using a bone reamer under fluoroscopic guidance, and confronted the risk of injury to the exiting and traversing nerve root. In very High-grade downward migration where the disc fragments are hidden in the rear of the pedicle i.e. the ruptured fragments lie in close contact with the medial wall of the pedicle, an alternative technique known as extended foraminoplasty is necessary. It consists of removing the upper and medial part of the pedicle along with superior facet undercut (foraminoplasty with oblique pediculectomy).
Highly migrated herniations are in some case multi-fragmented. There is a possible chance of remnants which can lead to unsuccessful disc fragments removal. But the foraminoplasty technique can give an assurance of a complete removal of the disc material. This technique is useful in treating downward highly migrated disc herniation. Lee et al. proposed an Epiduroscopic approach to help surgeons ascertain that the fragments are successfully removed [22].
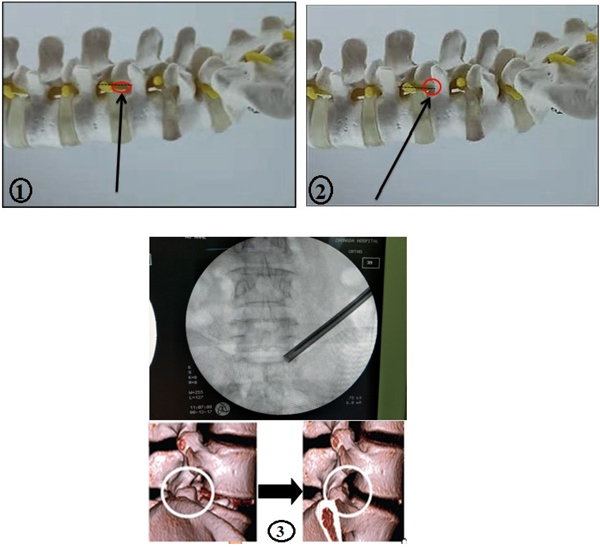
Lee and colleagues [55] introduced a new technique known as foraminoplastic superior vertebral notch approach FSVNA (Fig. 8.1-3). This technique is different from the conventional foraminoplasty in numerous aspects. The conventional foraminoplasty is an unstable reaming technique which allows the shifting of the guiding tube’s position into the cranial area of the foramen during the procedure with the risk of ineffective reaming and upper root injury [55]. Whereas, the FSVNA allows the procedure to be stable, without slipping and shifting of the reaming axis. The guide rod and the tube are docked between the Superior Articular Process (SPA) and the lower vertebral body. This stable reaming technique allows the endoscope to be placed dorsally close to the epidural space and more caudally far from the upper root, leading to a minimal risk of interruption of disc structure. The current technique is more advantageous compared to the conventional one, which is considered to be very challenging, expensive and time-consuming. The FSVNA is simple, cost-saving, and takes a very small amount of time. Another advantage of the FSVA is that it reduces the approach-related complications. However, there are several concerns in the current approach such as bleeding, neural injury caused by the blind approach this technique; it is performed under C- arm guidance.
Recently the transforaminal approach has been modified into a very promising technique known as the targeted fragmentectomy. This technique aims to only remove the migrating fragment without damaging the central disc [46]. This new technique can be applied to treat extruded lumbar disc herniation particularly for highly migrated disc herniation. It is performed via Transpedicular approach, Transarticular approach (via the superior articular process), Epiduroscopic approach, and extraforaminal approach.
12. INTERLAMINAR APPROACH
Lumbar disc herniation can in some cases be inaccessible with the transforaminal approach at L5-S1 level due to anatomical limitations such as in patients with a high iliac crest, a large facet joint or a small intervertebral foramen [53, 54]. However highly migrated lumbar disc herniation is also another issue that restricts the transforaminal approach, even with a curved probe [43, 55].
The Interlaminar approach (Fig. 9) allows an easy access into the L5–S1 level where the interlaminar window is naturally very large. This approach can also be applied in some cases at L4 –L5 level and is reported to be effective. Although this approach tried to overcome anatomical constraints at the L5- S1 level, the approach itself has limitations and needs alternative or additional techniques in order to successfully remove the disc fragments. A narrow interlaminar window can increase the difficulty and risk of nerve roots, cauda equina, and vascular injuries [56]. In this case, additional techniques such as endoscopic bone work and medial facetectomy are necessary in order to create enough space or enlarge the interlaminar space and avoid unnecessary damage. Numerous studies have reported this approach to be effective for highly migrated disc herniation in both cranial and caudal. [56, 53, 54]
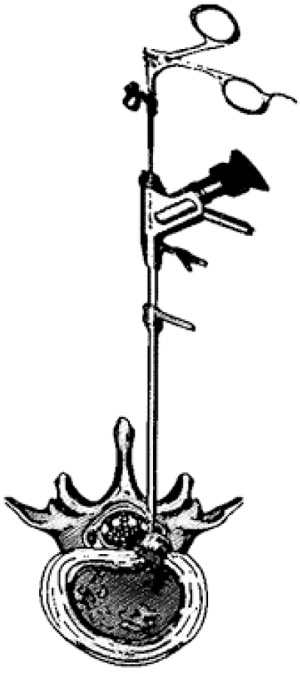
13. CONTRALATERAL APPROACH
PELD treatment for migrated disc herniation via the contralateral route is an alternative technique introduced by Kim et al. [60] The conventional PELD route can in some cases be obstructed due to anatomical barriers such as narrow ipsilateral intervertebral foramen and the pedicle. For these reasons, the ipsilateral transforaminal route is inaccessible. Even though combining foraminoplasty to this approach can help resolve these issues and give better access and visualization of the target. However, it is technically demanding and time-consuming. There may also be a higher risk of nerve injury and could cause excessive bone bleeding which cannot be controlled by radiofrequency coagulation. [57-59] The contralateral approach allows surgeons to have a wider angle in accessing the disc fragments of an extruded disc and a distal removal, especially in downward highly migrated disc herniation.
14. SURGICAL APPROACH SELECTION
Endoscopic spine surgeries for treating extruded disc herniation, particularly in highly migrated disc herniation is known to be very challenging and technically demanding. However, several studies have reported effective techniques with remarkable Outcomes. In the last few years, an increase of spine surgeries approaches has been proposed in order to overcome various issues encountered in endoscopic spine surgery. However surgical approach selection for the treatment of extruded lumbar disc herniation is based on many factors such as anatomical structures, availability of surgical instrument, the location of the disc herniation etc. All the approaches are feasible at all the lumbar spinal levels. Concerning the approach selection, some preoperative aspects such as physical examination, radiological findings, the expense of the surgery, surgeon experience, and surgical risk i.e. advantages and disadvantages of each approach should be taken into accounts.
A careful selection of procedure is essential to ensure favorable outcomes with minimal morbidity. Patient’s long-term postoperative satisfaction is important. It is hard to predict long-term outcome acknowledging that there are factors such as recurrence, surgery-related- complication, and other unforeseen events that can contribute to surgical failures. Additionally, doctor-patient communication may also play an important role in preoperative planning. Regarding the selection of a suitable surgical approach, one should consider the safest, efficient, least time-consuming and appropriate one; conventional open surgery should be considered for some difficult cases.
CONCLUSION
PELD techniques for treating extruded lumbar disc herniation, especially in migrated or sequestered ones are safe and effective, even though the choice for a suitable surgical approach is based on factors such as anatomical barriers, material availability, cost, the disc herniation location, and the surgeon’s experience. Local anesthesia is an important component of the procedure as this allows the patient to report any discomfort indicating a threat to the nerve roots. The probable causes of surgical failure include unsuccessful disc fragments removal, poor visualization, inexperienced surgeon, anatomical barrier, and factors related to the surgery or patient. Further research is needed in order to improve PELD techniques and help surgeons overcome issues encountered during the endoscopic spine surgeries.
DISCLOSURES
This study did not receive any financial or research support from any organization or institution.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest. The present study has not been presented or submitted elsewhere.
ACKNOWLEDGEMENTS
Declared none.


