All published articles of this journal are available on ScienceDirect.
Nerve Palsy after Total Hip Arthroplasty without Subtrochanteric Femoral Shortening Osteotomy for a Completely Dislocated Hip Joint
Abstract
Background:
Neurological injuries are a rare but devastating complication after total hip arthroplasty (THA). The purpose of this study was to retrospectively determine the frequency of nerve palsy after THA without subtrochanteric femoral shortening osteotomy in patients with a completely dislocated hip joint without pseudo-articulation between the femoral head and iliac bone.
Methods:
Between October 1999 and September 2001, nine primary THAs were performed for patients with a completely dislocated hip joint. The limb lengths, neurological abnormalities, and the extent of their neurological recovery were evaluated. Three THAs were combined with subtrochanteric femoral shortening osteotomy, and six THAs were combined without subtrochanteric femoral shortening osteotomy.
Results:
The mean length of the operation was 4.8 cm (range, 3.0-6.5 cm). Sciatic nerve palsy developed in four of the nine patients after THA. None of the cases with sciatic nerve palsy were combined with subtrochanteric femoral shortening osteotomy. Three of four patients did not completely recover from sciatic nerve palsy.
Conclusions:
THA for patients with a completely dislocated hip was associated with a high risk of nerve palsy due to excessive limb lengthening; the potential for recovery from nerve palsy was observed to be poor. Subtrochanteric femoral shortening osteotomy should be used in combination with THA in patients with a completely dislocated hip.
INTRODUCTION
Nerve palsy is an uncommon but acknowledged complication of total hip arthroplasty (THA) [1]. The incidence of nerve palsy after THA has been reported to range from 0.08%-3.7% [2-8]. Several lines of evidence have confirmed that limb lengthening is an important factor in the development of nerve palsy [5, 7-10], especially in patients with developmental dysplasia of the hip (DDH) [3-7]. Subtrochanteric femoral shortening osteotomy is therefore regarded as an essential surgical technique that should be performed with THA in patients with a highly dislocated hip joint [11]. In particular, in a completely dislocated hip joint without pseudo-articulation between the femoral head and iliac bone, subtrochanteric femoral shortening osteotomy is needed because the acetabular component should be placed in the true acetabulum, where it provides a better bone stock [12].
However, the frequency with which nerve palsy develops after THA without subtrochanteric femoral shortening osteotomy for patients with a completely dislocated hip joint without pseudo-articulation between the femoral head and iliac bone is unknown. Thus, the aim of the present was to determine the frequency of nerve palsy after THA without subtrochanteric femoral shortening osteotomy in patients with a completely dislocated hip joint without pseudo-articulation between the femoral head and iliac bone.
The study protocol adhered to the ethical guidelines of the 1975 Declaration of Helsinki. The institutional review board of the Faculty of Medicine, Saga University at Saga, Japan, approved this study.
MATERIALS AND METHODS
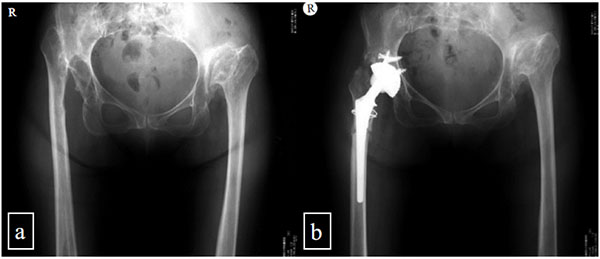
We performed a retrospective study of all of the patients with a completely dislocated hip joint without pseudo- articulation between the femoral head and iliac bone who underwent THA between October 1999 and September 2001. In total, we performed THA operations on six hips in six patients without subtrochanteric femoral shortening osteotomy (Fig. 1). In same the period, three hips in three patients received THA combined with subtrochanteric femoral shortening osteotomy according in accordance with the procedure described by Sonohata [12] (Fig. 2). These three hips were used as controls.
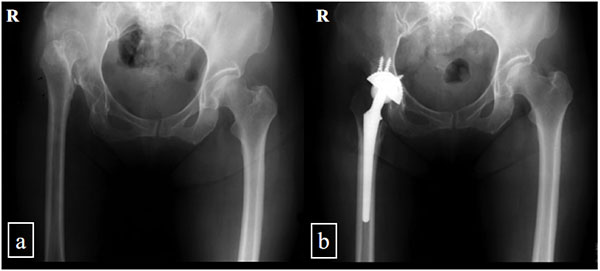
Nine hips in nine patients (7 females, 2 males) were included in this study. The patients had no history of nerve trauma, neurological disease, or pelvis or femoral osteotomy. The average age of the patients at the time of the operation was 58 years (range, 49-66 years). The average duration of follow-up monitoring was 135 months (range, 18-1213 months) (Table 1).
| Case | Osteotomy | Age of THA | Sex | Height (cm) | Weight (Kg) | BMI (Kg/m2) | Opposite hip joint | Follow-up period (month) |
|---|---|---|---|---|---|---|---|---|
| 1 | - | 51 | Female | 158.5 | 54 | 21.4 | OA* | 181 |
| 2 | - | 49 | Male | 168.5 | 49.4 | 17.2 | normal | 193 |
| 3 | - | 63 | Female | 140.5 | 55 | 27.7 | OA** | 181 |
| 4 | - | 66 | Female | 148 | 66 | 30.1 | OA*** | 145 |
| 5 | - | 65 | Female | 139 | 47 | 24.3 | normal | 76 |
| 6 | - | 58 | Female | 147 | 49 | 22.7 | normal | 18 |
| 7 | + | 56 | Female | 142 | 41 | 20.3 | OA*** | 203 |
| 8 | + | 52 | Female | 145 | 41 | 19.5 | OA*** | 182 |
| 9 | + | 63 | Male | 150 | 66 | 29.3 | ankylose | 34 |
| Average (mean ± SD, range) | 58±6, 49-66 |
149 ±10, 139-169 |
52 ±9, 41-66 |
24 ±5, 17.2-30.1 |
135±72, 18-1213 |
The indications for surgery were severe hip pain and/or considerable difficulty walking and performing daily activities. All of the operations were performed by a single surgeon. The decision to perform femoral shortening osteotomy was made by the operator. Six patients received only spinal anesthesia, one patient received only epidural anesthesia, and two patients received spinal and epidural anesthesia, based on the decision of the anesthesia team.
All of the operations were performed using the posterolateral approach with a cementless femoral component (PerFix-HA femoral component; Kyocera, Kyoto, Japan) with a 22- or 28-mm alumina or zirconia ball and an AMS-HA acetabular shell with an ABS (alumina ceramic inlay mechanically fixed to a polyethylene liner) or an AMS (polyethylene liner) liner. Whether or not the operator checked the tension of the sciatic nerve under direct vision is unclear.
A routine radiographic examination including anteroposterior and frog-leg lateral radiographs was performed. On the anteroposterior radiographs, a line was drawn through the teardrops, and the perpendicular vertical distance of the apex of the lessor trochanter from this reference line was compared to identify leg length discrepancy and leg lengthening [13]. The acetabular components were evaluated at the most recent follow-up for evidence of migration, in accordance with the method of Carlsson and Gentz [14]. The bone-metal interface was evaluated at the most recent follow-up to identify the presence and progression of radiolucent lines in the three zones described by DeLee and Charnley [15]. The femoral component was evaluated for changes in the position, subsidence, and radiolucency in the seven zones described by Gruen et al. [16]. The stability of the femoral component was assessed as bone-ingrown fixation, stable fixation, or unstable fixation in accordance with the fixation/stability score described by Engh et al. [17]. The orientation of the component was classified as valgus, slightly valgus, neutral, slightly varus, or varus, in accordance with the classifications of Christie et al. [18]. Slightly valgus or slightly varus alignment was used to describe a femoral stem with less than 5° of malalignment with respect to the neutral axis of the femoral canal. Varus or valgus alignment was used to describe a femoral stem that was oriented ≥5° beyond the neutral position.
The height of the hip center was measured as the perpendicular distance from the inter-teardrop line. A high hip center was defined as a hip with a center located >35 mm proximal to the inter-teardrop line [19].
All of the hips were evaluated using the Japanese Orthopaedic Association (JOA) Hip Score. The JOA score consisted of four categories, with a maximum total score of 100 points: pain (40 points), range of motion (20 points), walking ability (20 points), and activities of daily living (20 points).
The mean JOA scores at the preoperative and final follow-up examinations and the preoperative and postoperative leg length discrepancy were compared using the paired t-test. The JOA score was statistically evaluated in patients with over five years of follow-up. All of the statistical analyses were performed using the software program SPSS for Windows (Version 22; IBM Corp, Armonk, NY, USA). P values <0.05 were considered to indicate statistical significance.
RESULTS
Clinical Results
The average duration of follow-up was 135 months (range, 18-1213 months). The average JOA hip score for the patients improved from an average of 54 (range, 22-76) preoperatively to 77 (range, 60-99) postoperatively (p<0.05) (Table 2).
The mean outer diameter of the acetabular components was 45 mm (range, 42-46 mm). Five AMS-HA acetabular shells with an ABS (alumina ceramic inlay mechanically fixed to a polyethylene liner) liner were used with a 28-mm alumina ball. Four AMS-HA acetabular shells with an AMS (polyethylene liner) liner were used with a 22-mm zirconia ball. The average number of screws was 3.5 (3 in 6 hips, 4 in 1 hip, 5 in 2 hip) for each of the acetabular cups. The average distal diameter of the stems was 9.8 mm (9 mm in 3 hips, 10 mm in 5 hips, 11 mm in 1 hip). A bone graft to the acetabulum was performed in four hips (block in three hips, morselized bone in one hip). A bone graft to the femur was performed in three hips at the site of subtrochanteric osteotomy. Liner and ball revision surgery for the ABS acetabular component failure was performed for all ABS liners [20]. Stem revision surgery for sciatic nerve palsy was performed seven days after primary THA in one hip. Cup revision surgery for cup loosening was performed 30 months after primary THA in 1 hip. Two hips had an intraoperative greater trochanteric fracture that necessitated additional wiring.
| Case | JOA hip score (before operation) | JOA hip score (last follow-up) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain | ROM | Gait | ADL | Total | Pain | ROM | Gait | ADL | Total | |
| 1 | 20 | 16 | 10 | 10 | 56 | 35 | 19 | 5 | 10 | 69 |
| 2 | 30 | 16 | 5 | 18 | 69 | 40 | 19 | 20 | 20 | 99 |
| 3 | 10 | 13 | 10 | 18 | 51 | 40 | 15 | 18 | 12 | 85 |
| 4 | 20 | 18 | 10 | 12 | 60 | 40 | 17 | 5 | 8 | 70 |
| 5 | 20 | 15 | 10 | 14 | 59 | 35 | 17 | 10 | 12 | 74 |
| Average (mean ± SD, range) | 20±7, 10-30 |
16±2, 13-18 |
9±2, 5-10 |
14±4, 10-18 |
59±7, 51-60 |
38±3, 35-40 ** |
17±2, 15-19 NS |
12±7, 5-20 NS |
12±5, 8-20 NS |
79±13, 69-99 * |
*, p<0.05; **, p<0.01; NS, no significant difference.
Radiographic Results
The average horizontal distance of the center of the femoral head was 31 mm (range, 25-42 mm), and the average height of the hip center was 25 mm (range, 11-38 mm). One hip (Case 1) was considered to have a high hip center.
The average leg length discrepancy of the patients improved from an average of 5.6 mm (range, 3.0-7.5) preoperatively to 1.0 mm (range, 0-3.0) postoperatively (p<0.01). The average increase in the leg length was 4.8 mm (range, 3.0-6.5) (Table 3).
| Case | Osteotomy | Operation time(min) | Total blood loss(g) | Preoperative leg length discrepancy (cm) | Leg lengthening(cm) | Postoperative leg length discrepancy (cm) | Perpendicular distance between the cup center and the inter-teardrop line (mm) |
|---|---|---|---|---|---|---|---|
| 1 | - | 75 | 1434 | 7.5 | 6.5ψ | 1.0ψψ | 38 |
| 2 | - | 145 | 1717 | 5.5 | 4.5 | 1.0 | 22 |
| 3 | - | 85 | 896 | 6.0 | 5.5 | 0.5 | 20 |
| 4 | - | 85 | 660 | 6.0 | 6.0 | 0 | 11 |
| 5 | - | 80 | 1067 | 6.5 | 6.0 | 0 | 21 |
| 6 | - | 85 | 173 | 4.5 | 4.5 | 0 | 23 |
| 7 | + | 130 | 830 | 3 | 4 | 1 | 33 |
| 8 | + | 215 | 1427 | 6 | 3 | 3 | 34 |
| 9 | + | 145 | 1699 | 5 | 3 | 2 | 20 |
| Average (mean ± SD, range) | 116±47, 75-215 |
1100±516, 173-1717 |
5.6±1.3, 3.0-7.5 | 4.8±1.3, 3.0-6.5 | 1.0±1.0, 0.0-3.0** | 24.7±8.6, 11-38 |
All of the stems were in the neutral position. Two acetabular components migrated (Cases 1 and 7); while the Case 1 did not require revision surgery, Case 7 require did. The other components showed no evidence of migration or loosening. No femoral or acetabular components displayed radiolucency. There were no cases of subsidence in the femoral component. Fixation was achieved in all of the femoral components with an optimal interface at the latest follow-up. One case (Case 1) required femoral revision surgery to achieve relaxation of the sciatic nerve to treat sciatic nerve palsy.
Complications
There were no cases of infection. During the operation, two greater trochanteric fractures occurred, and additional wiring was performed. Dislocation occurred in five hips (Cases 1, 4, 7, 8, and 9) within two weeks after THA. Case 4 required open reduction.
There were 4 cases of neurologic abnormalities. In Cases 2 and 3, a physical examination of the motor and sensory functions along the femoral and sciatic nerves revealed no changes between the preoperative values and the most recent follow-up. In Case 1, the patient experienced numbness in her right lower legs and foot one day after THA. The muscle strength in her right ankle and toes was rated as grade 0 (maximum 5). One week after THA, stem revision was performed to reduce the tension of the soft tissue around her right hip joint. At the last follow up, she had light paresthesia in her right lower leg; the sensory disturbance in the dorsal foot and plantar foot was classified as grade 8 (maximum 10) and grade 2, respectively. Her muscle strength improved to grade 1 in dorsal flexion and grade 2 in plantar flexion. In Case 4, the patient experienced hypesthesia in her left plantar foot and severe pain on the lateral side of her left thigh, which was induced by knee extension one day after THA. Fortunately, she had no motor dysfunction. At the last follow up, she complained of mild sensory disturbance and pain. Case 5 experienced numbness in the right proper sensory area of the deep peroneal nerve and sensory disturbance in the dorsal foot and plantar foot of grade 5-6 (of 10) and grade 2-3, respectively. The muscle strengths of her right extensor hallucis longus, tibialis anterior, and gastrocnemius muscle were grade 0 (of 5), grade 1, and grade 2, respectively. At the last follow up, the sensory disturbance in the right proper sensory area of her deep peroneal nerve improved to grade 3 (of 10); however, there was no remarkable recovery in her dorsal or plantar foot. The muscle strength of her right extensor halluces longus, tibialis anterior and gastrocnemius muscle improved to grade 2 (of 5), 3, and 4, respectively. In Case 6, the patient experienced paresthesia in the right sensory area of the femoral and sciatic nerves; however, the muscle strength in her right lower limbs was not disturbed. Her neurologic abnormality completely recovered within two months after surgery (Table 4).
| Case | Osteotomy | Complications | Treatment | Follow-up period (month) | Outcome | Other operation |
|---|---|---|---|---|---|---|
| 1 | - | Incomplete paralysis of the sciatic nerve, acetabular component migrated, dislocation |
Stem revision | 181 | Residual paralysis (partial recover) | Liner and ball revision for liner dissociation (6 years after THA) |
| 2 | - | Trochanteric fracture during operation | Wiring | 193 | Union | None |
| 3 | - | None | 181 | Liner and ball revision for liner dissociation (10 years after THA), right total knee arthroplasty, left unicompartmental knee arthroplasty |
||
| 4 | - | Incomplete paralysis of the sciatic nerve, dislocation |
Surgical reduction | 145 | Residual paralysis (partial recover) | Open reduction for dislocation (7 days after THA), liner and ball revision for liner dissociation (3 years after THA) |
| 5 | - | Incomplete paralysis of the sciatic nerve | Observation | 76 | Residual paralysis (partial recover) | None |
| 6 | - | Incomplete paralysis of the sciatic and femoral nerve, | Observation | 18 | Complete recovery | None |
| 7 | + | Fracture of proximal fragment during operation | Wiring | 203 | Union | Cup revision for loosening after 30 months of primary THA |
| 8 | + | Dislocation | Observation | 182 | stabilized | None |
| 9 | + | Dislocation | Observation | 34 | stabilized | None |
DISCUSSION
Clinical neurological deficits after THA are relatively uncommon, but they can be highly problematic to the patient and surgeon when they occur. However, how to determine the maximum extent of leg lengthening that can be expected without causing neurologic complications is unclear [21].
The mean increase in the leg length in the 9 legs of our series was 4.8 cm, and nerve palsy occurred after THA in 4 cases (44%). All four cases underwent THA without subtrochanteric femoral shortening osteotomy. The increase in leg length in the 4 paralyzed cases was from 4.5 cm to 6.5 cm, and complete recovery was achieved in only 1 case who had 4.5 cm leg lengthening. In contrast, the increase in leg length in the non-paralyzed case was from 3.0 cm to 5.5 cm. This is the first report to investigate nerve palsy in patients undergoing THA without subtrochanteric femoral shortening osteotomy for a completely dislocated hip joint. An adverse event that occurs in 4 out of 6 cases can be described as extremely frequent. Sonohata et al. [12] reported that there were no cases of nerve palsy after THA combined with subtrochanteric shortening osteotomy in patients with a completely dislocated hip joint. In the current study, THA without subtrochanteric femoral shortening osteotomy for a completely dislocated hip joint was found to be a high-risk operation and a plausible etiology of nerve palsy. Thus, it should be essential to perform subtrochateric shortening osteotomy in patients with a completely dislocated hip joint who are undergoing THA.
Previous studies have demonstrated a difference in the prognosis for recovery. The gradual recovery seen in many patients with sciatic nerve injury has been well documented in the literature. The incidence of permanent, disabling nerve damage is only about 25% [10, 22, 23]. Neverthless, Ferrell et al. [21] noted that in the majority of patients with nerve palsy, whether complete or incomplete, the strength never fully recovered to the preoperative level. In our patients, only 1 patients achieved complete recovery, and her leg lengthening was 4.5 cm. However, three patients had not completely recovered by the time of the final follow-up examination, supporting the above-mentioned hypothesis.
The present study is associated with several limitations. First, the study group only included 9 cases and was relatively small. Thus, risk factors such as gender, the approach, and the use of a cementless stem were not investigated. Second, the measurement of nerve palsy was not standardized, because this study was retrospective.
The strengths of this study include its focus on THA in a completely dislocated hip joint. All of the cases had a uniform pathology with the potential for nerve palsy after THA. Our findings emphasize the need for subtrochanteric femoral shortening osteotomy in order to safely perform THA in patients with a completely dislocated hip. The results of the present study also support the notion that patients with nerve palsy associated with THA do not fully recover [21].
CONCLUSION
In THA for patients with a completely dislocated hip, it is possible to place an acetabular shell at the true acetabulum using the usual surgical technique. However, this is associated with a high risk of nerve palsy due to excessive limb lengthening. Furthermore, nerve palsy shows a poor potential for recovery. We believe that subtrochanteric femoral shortening osteotomy should be used in combination with THA for patients with a completely dislocated hip.
No benefits in any form were received or will be received from a commercial party related directly or indirectly to the subject of this article.
LIST OF ABBREVIATIONS
| DDH | = Developmental dysplasia of the hip |
| JOA | = Japanese Orthopaedic Association |
| THA | = Total hip arthroplasty |
CONFLICT OF INTEREST
The authors did not receive and will not receive any benefits or funding from any commercial party related directly or indirectly to the subject of this article.
ACKNOWLEDGEMENTS
The authors thank Dr. Takao Hotokebuchi for his contributions to this study.


