All published articles of this journal are available on ScienceDirect.
A Qualitative Description of Chronic Neck Pain has Implications for Outcome Assessment and Classification
Abstract
Background:
Neck pain is common, but few studies have used qualitative methods to describe it.
Purpose:
To describe the quality, distribution and behavior of neck pain.
Methods:
Sixteen people (15 females; mean age = 33 years (range = 20-69)) with neck pain >3 months were interviewed using a semi-structured guide. Interview data were recorded and transcribed verbatim. Descriptive content analysis was performed by two authors. Participants then completed an electronic descriptive pain tool, placing icons (word and icon descriptors to describe quality) on anatomic diagrams to identify location of pain, and intensity ratings at each location. This data was triangulated with interviews.
Results:
Aching pain and stiffness in the posterior neck and shoulder region were the most common pain complaints. All patients reported more than one pain quality. Associated headache was common (11/16 people); but varied in location and pain quality; 13/16 reported upper extremity symptoms. Neuropathic characteristics (burning) or sensory disturbance (numbness/tingling) occurred in some patients, but were less common. Activities that involved lifting/carrying and psychological stress were factors reported as exacerbating pain. Physical activity was valued as essential to function, but also instigated exacerbations. Concordance between the structured pain tool and interviews enhanced trustworthiness of our results. Integrating qualitative findings with a previous classification system derived a 7-axis neck pain classification: source/context, sample subgroup, distribution, duration, episode pattern, pain/symptom severity, disability/participation restriction.
Conclusions:
Qualitative assessment and classification should consider the multiple dimensions of neck pain.
INTRODUCTION
It is estimated that one-third to one-half of all adults will experience neck pain (NP) during the course of one year [1, 2]. Whiplash injury is the most common traumatic cause of NP [3]. NP may be associated with degenerative changes, physical, and psychosocial factors [4-6] and is typically episodic [3, 7-9]. There is a substantial risk of developing chronic symptoms that cause disability, work loss, and health care costs [7, 10]. Structural abnormalities found in diagnostic imaging are weakly associated with intensity and prognosis of NP due to injury [11-13], or chronic neck dysfunction [11]. As a result, neither diagnosis nor classification on the basis of imaging is possible.
NP is a generic term that can encompass a variety of anatomic and pathological impairments. The Bone and Joint Decade Task Force on Neck Pain observed that “neck pain may be a feature of virtually every disorder and disease that occurs above the shoulder blades” and confined itself to “pain located in the anatomic region of the neck…, with or without radiation to the head, trunk, and upper limbs” [14]. This is sometimes also referred to as “nonspecific neck pain”.
The Neck Pain Task Force suggested a classification system for NP based on a synthesis of the literature including pre-existing classification systems [14]. The result was a classification system based on five axes: two related to context and three related to the nature of the NP (severity, duration and pattern). Severity was classified into four categories that integrated intensity (of symptoms) and disability: grade I, low disability–low intensity; grade II, low disability–high intensity; grade III, high disability–moderately limiting; and grade IV, high disability–severely limiting [14]. Duration was defined as: transitory, short duration, or long duration. The Pattern was described as: single episode, recurrent, or persistent. This classification system did not incorporate qualitative methodologies, nor did it consider the qualitative nature of the pain as a necessary feature of taxonomy.
When assessing pain in people with neck disorders, the focus is typically on pain intensity. There is emerging evidence in traumatic NP that the quality of the pain influences prognosis, treatment requirements and response [15-18]. Recognizing the complex nature of NP, the Neck Pain Task Force suggested that future NP research should embrace both quantitative methods and qualitative approaches to more fully explore the nature of this complexity [14]. Qualitative methods do not attempt to test hypotheses, but rather to provide a richer description or understanding of a phenomenon. A better understanding of NP could inform practice, determine if outcome measures accurately reflect NP or describe dimensions of NP that should be studied in future quantitative studies.
The purpose of this study was to describe the pain experience of people with nonspecific NP using a qualitative descriptive approach. A secondary purpose was to propose how this information might be useful to update a descriptive classification to better reflect the patient perspective.
MATERIALS AND METHODS
Design: Qualitative Description
A descriptive approach is used in qualitative research when there is a need for basic qualitative description of phenomena, as opposed to other qualitative paradigms that might involve interpretation or theory. While it might be assumed we know what NP is, qualitative approaches allow us to explore these assumptions and understand how variable it is. Describing the pain experience in “concrete everyday language” is consistent with the descriptive approach recommended by Sandelowski [19, 20]. The primary purpose of this study was to describe NP from the perspective of individuals who are currently experiencing the condition.
Subjects
Participants were recruited through study flyers posted on the university campus, and from a database of patients who had participated in previous neck studies. The eligibility criteria were: 1) 18 years and older; and 2) had NP of any cause, with or without arm/shoulder pain, chronic or recurrent (recurrent being more than one episode in the past 3 months).
Procedure
To identify the experience of NP, 16 semi-structured interviews were conducted face-to-face and recorded for the purpose of analysis. Two researchers with prior interview experience conducted the qualitative interviews using a semi-structured interview guide developed to elicit the participant's description of the nature of their pain and its behavior. The study was approved by the McMaster Research Ethics Board and all participants provided informed consent.
Interviews were followed by administration of a structured, icon-based, elicitation tool that collects quantitative information on pain quality, intensity and location. Both data sets were independently analyzed and then compared to triangulate the validity of the pain information.
Descriptive Interviews
Interviews (see Table 1 for interview guide) were designed to identify the painful symptoms experienced by the respondents, and to describe related patterns/behavior. All questions were vetted by an expert committee and trialed on practice respondents prior to initiation of the current study. The study was not designed to study the overall impact of NP on function or quality of life, but the interviewers asked the respondents probing questions if activity was cited as a factor influencing NP. All the interviews were audio-taped and transcribed verbatim.
| Onset |
| When did your neck problems start? |
| Symptoms |
| What symptoms do you have with your neck problem? |
| If you have pain, what does the pain feel like? Can you use words to describe it? |
| What is the intensity of your symptoms? |
| Do you notice any patterns to your symptoms? |
Structured Icon-based Elicitation
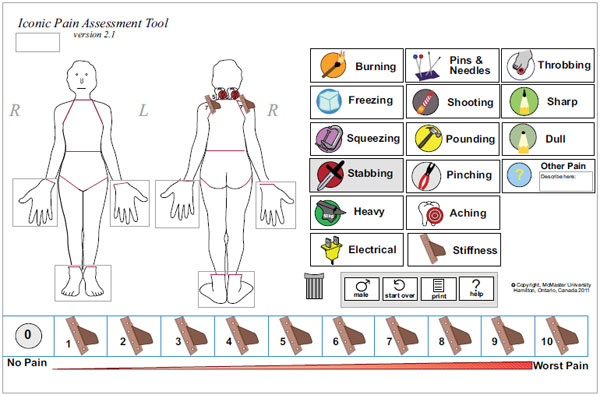
Participants also completed a structured, electronic tool [21-25] where respondents select icons with associated words (total of 16 word options) that reflected the qualitative nature of their pain. The participant placed icons on the affected locations of their body diagram and then rated the severity (0 = no pain, 10 = worst pain) at each location. The tool was designed to be web-based, but can be used as a print version. The tool has undergone field-testing and evaluation and has been found to be a user-friendly method to communicate the pain experience in both adults and children [21-25]. The iconic pain evaluation was performed after the interviews to avoid biasing participants.
Analysis
The recorded interviews were transcribed verbatim and analyzed using content analysis within the descriptive tradition [19, 20]. The analysis followed six phases: familiarizing with data, generating initial codes, searching for themes, reviewing themes, defining and naming themes, and reporting the analysis of the findings.
The transcribed interviews were reviewed by two researchers who independently coded the interview transcripts. Researchers read transcripts for overall content and then reviewed text in detail to assign initial codes. Codes were grouped, collapsed and developed into themes. Illustrative quotes were selected to demonstrate themes and represent different respondents. The researchers met to arbitrate any disagreement with coding/themes, and to agree on the subset of quotes that reflected the themes. The iconic data were summarized descriptively.
The factors identified by participants in this study were compared to existing classification systems, and a descriptive classification system was proposed and reviewed by a multidisciplinary, international panel of expert clinicians involved in the management of NP.
Trustworthiness of the Data/Findings
To verify the trustworthiness of the data, the identified themes and quotes were also reviewed with the study participants (“member checking”). Feedback was requested, both in terms of whether the existing themes accurately reflected the participants’ experiences, and to identify themes that may be missing. The draft manuscript was also reviewed by clinicians (7 physiotherapists, 3 chiropractors, 2 physicians, 1 psychologist) and individuals (2 university employees) with NP outside of the study respondents. Data quality was also confirmed by ensuring that descriptors selected on the iconic tool were in agreement with descriptions in the open-ended qualitative interview.
RESULTS
A total of 16 participants with NP for more than 3 months’ duration were interviewed (Table 2). Twelve were university students with a minimum annual household income over $40,000. Four other participants were employed or retired (salary range = $20,000-$99,999). Member checking resulted in complete agreement with the themes identified and their content.
| Demographic | |
|---|---|
| N | 16 |
| Age* (range) | 33 (20-69) |
| % Female | 94% |
| Symptom duration* (range) | 10.4 (0.5-53.0) |
| % Participants with history of whiplash | 19% |
| % University educated | 88% |
Themes around the pain experience included the anatomic distribution of the pain, the quality and intensity of the pain, the behavior of the pain, mediators of the pain, and impact on activity.
The Anatomic Distribution of Neck Pain
All participants reported pain in the neck region. The area identified was consistent with the area defined as NP by the Neck Pain Task Force involving the occiput to shoulder area posteriorly [14]. A majority (n=11) of the respondents, including all of those with a history of whiplash, had headache associated with their NP. The location and quality of headache varied. Some described this as a “migraine”, whereas others specified a location (e.g., “temple”). The location of headache was not consistent across participants, e.g., one reported a “Really bad headache, a dull ache at the back of my head”; while another described “pain up through just the left side of my head that sort of shoots up there”. For a minority of participants (n=3), headache was their predominant complaint, exceeding the discomfort of their NP.
A number of participants reported upper extremity symptoms—most commonly the shoulder (n=13). The pain was often diffuse through their neck and shoulder, whereas the minority (n=3) indicated a specific area such as “the collarbone area, at the back”. Some participants reported widespread pain problems, including “upper back pain through to my chest, to shoulder pain and down my arms”.
The Quality and Intensity of Neck Pain
Almost every participant reported more than one type of pain and was able to differentiate different pains in terms of their qualitative nature. Further, different pain quality types usually had different pain intensities and behaviours. NP was most commonly described as persistent, dull and achy. This pain was typically of moderate intensity, or 3-6 range on a numeric 11-point pain rating scale, and was usually persistent or “always there to some extent”. Respondents reported pain overlay consisting of momentary to more prolonged episodes of acute pain. Very brief, but intense symptoms were noted by a number of participants. In some cases, this brief sharp pain was described as “piercing” or “stabbing”. One participant, with intense pain following her whiplash injury, described her NP as “burning, stinging, raw, deep pain”. Some participants experienced a complex mixture of painful symptoms:
“It’s a dull throbbing ache most of the time. And then, it almost feels like it spasmed up, it’s like a burning hot poker and that’s when it goes right through …to my tongue; even it gets affected. It almost goes into a numb state. It’s like I can’t move for a second or two.”
Pain quality often interacted with temporal and spatial qualities as explained by one participant who stated, “it tenses right up, and it goes from the neck the back of the head… and later I get the headache”.
The pain that occurred beyond the “usual” aching pain was always characterized as being more intense or bothersome, and was typically rated as either a 9 or 10 in intensity by participants who reported these intermittent episodes.
Other Bothersome Symptoms
Participants reported other bothersome symptoms that would not be considered as fitting the classic definition of pain. The most common of these symptoms were “tightness” or “cramping”. The spasm subtype of symptoms was usually in the posterior neck and shoulders. A minority of participants noted symptoms in their hands. Symptoms in the hand were more neurological in nature and included “shocks”, “pins and needles”, and “numbness”. One participant noted “clicking” in the neck with movement.
The Behavior of Neck Pain and its Mediators
Participants reported three different categories of factors that affected their NP: position/posture, activity and stress. Neck postures that require flexion or extension, such as looking down when reading a book, and looking up, were postures that were most often reported as increasing symptoms: “When I’m reading a book, I usually hold it up in front of my face so I don’t lean over a desk to read it. If I’m reading the paper, I have to be careful of what angle I read the newspaper … to reduce pain.”
A number of participants stated that they had become aware over time that the postural alignment of their lower spine affected their neck pain (n=5) as exemplified by the person who stated “whenever my back hurts, it makes my neck hurt.”
A number of the participants noted that psychological stress (n=6), including “rushing,” increased their pain: “If I am more stressed, then it is sometimes ten. If it is an okay day…then it is maybe five.” This was also illustrated by another participant who stated “I think that it is all about whatever is in your mind; what causes you to stress out, that is what causes your body to ache.”
Most participants did not relate their pain to a time of day, but rather to the postural, movement, or stress factors incurred during the day. This was clarified by a participant who suggested “I’d say probably (say worst) at the end of the night before I go to sleep. I find the pain of the day kind of catches up with me and all the stress of the day catches up with my body”. Conversely, a number of participants found the headache to be less predictable, experiencing “unpredictable and sudden enormous headache”.
Participants consistently noted that more vigorous activity or sustained postures exacerbated their pain. Activities that involved use of neck/shoulder muscles, such as lifting and carrying, were the most commonly mentioned activities that aggravated neck and shoulder pain.
The Complex Relationship Between Neck Pain and Activity
A recurrent theme emerged about the complex relationship between physical activity or exercise and NP. Participants noted that they had to give up valued activity because of their NP. This loss affected their life satisfaction, but they also expressed concern that reduced activity would have adverse health consequences in the longer-term. Many noted that overexertion increased their NP, although a number also recognized that if they did not “keep moving” or do their exercises, their NP and function worsened. Participants spoke about their struggle to maintain an active lifestyle against the background of their chronic pain. Many accepted that having pursued many avenues of treatment, it was unlikely that they would be “cured”. This motivated their desire to regain meaningful activities, particularly where these were beneficial for their overall health. Symptom interference with resumption of activity was a barrier to achieving their desired level of activity and function.
Quantitative - Descriptive Findings from the Iconic Tool
The iconic assessment allowed participants to provide structured responses on quality, intensity and location of pain. The majority of respondents identified regions in both the neck and shoulder as being painful—these are summarized separately in Table 3. The majority of participants (n=9/15) selected aching and stiffness as descriptors of their NP. Seven participants also experienced pain radiating down their arms, identified most commonly as either stiffness (n=2) or aching (n=2). When present, the symptom “pins and needles” was rated as having a high intensity (8/10). There was wide variability across participants in the location, range and intensity of pain symptoms.
Eight participants reported symptoms in the hands with pins and needles (n=5; intensity range=3-6/10) and aching (n=2; intensity range=4-5/10) being the most common icons selected to describe arm pain. Exemplar diagrams from 2 participants are presented in Fig. (1) (localized presentation) and Fig. (2) (diffuse presentation) to illustrate different patterns of neck pain.
| Icon Descriptor | Frequency (Number of participants who selected the quality icon to describe their pain) |
Intensity Median Score (Range) on a scale of 0-10, 0 is no pain and 10 is worst pain |
||
|---|---|---|---|---|
| Neck Area (n=15) |
Shoulder Area (n=14) | Neck Area (n=15) |
Shoulder Area (n=14) | |
| Aching | 9 | 8 | 4 (2-9) | 5 (2-8) |
| Stiffness | 9 | 4 | 5 (2-8) | 6 (3-8) |
| Burning | 4 | 3 | 8 (7-9) | 8 (3-9) |
| Stabbing | 2 | 2 | 6 (4-8) | 8 |
| Heavy | 2 | 1 | 8.5 (8-9) | 8 |
| Pinching | 2 | 1 | 5 (1-9) | 5 |
| Squeezing | 2 | 1 | 7.5 (7-8) | 7 |
| Pins and Needles | 1 | 1 | 8 | 8 |
| Shooting | 1 | 0 | 4 | N/A |

Five participants experienced pain in the head with pounding (n=3; intensity range=3-9/10) being the most common icon to describe headache pain.
Six participants also experienced back pain with aching (n=3; intensity range=4-8/10) and stiffness (n=4; intensity range=3-9/10) being the most common icon to describe the pain. Other pain areas were also reported by some patients that were not related to their neck, e.g., lower extremity: legs (n=1) and feet (n=1).
We then integrated our findings and prior axis-based classification to create a more comprehensive descriptive classification of NP as presented in Table 4. A version of this table with clarification of the coding is provided as an appendix.
| Descriptive Neck Pain Classification | ||||
|---|---|---|---|---|
|
Axis I Context |
Axis II Sample Subgroup |
|||
| □ General Population (Screening) | □ General Population □ Special population (e.g. sport, occupation) ____________ |
|||
| □ Clinical Setting (Treatment) | □ Emergency Room □ Primary Care □ Secondary Care/Rehab _____________ □ Tertiary/Specialty Care ___________ |
|||
| □ Claim (Compensation) | □ Care or equipment only (no loss time) □ Reduced/modified hours at work □ Off-work/Wage replacement □ Long-term disability; pain and suffering |
|||
|
Axis III Distribution |
Axis IV Duration |
Axis V Pattern |
||
| □ Grade 1 (Neck only): Symptoms localized to neck (occiput to T1) □ Grade 2 (Neck/shoulder): Symptoms localized to neck/shoulder region (occiput to inferior angle of scapula) □ Grade 3 (Neck-diffuse): Two or more of: 1. Headache 2. Neck/ shoulder 3. Hand/arm symptoms □ Grade 4 (Neck-neurological): Neck pain with neurological signs and symptoms _______________ □ Grade 5 (Neck-major pathology): Neck pain secondary to major pathology (e.g. fracture/dislocation) _____________________ |
□ Transitory (0-7d) □ Short Duration (7d-3M) □ Long Duration (>3M) |
□ Single episode □ Recurrent □ Persistent - stable □ Persistent - unstable |
||
|
Axis VI Neck Pain; Symptom Severity |
Axis VII Disability/ Participation restriction |
|||
| □ None □ Mild □ Moderate □ Severe Pain Measure ______________ Score _________ |
□ None □ Mild □ Moderate □ Severe Disability Measure _______________ Score __________ |
|||
| Notes: | Notes: | |||
DISCUSSION
A mixed-methods descriptive approach illustrated a spectrum of different presentations of NP from simple to diffuse based on different factors that contribute to the nature and behavior of pain episodes experienced. The clarity with which participants described different pain types suggested that pain quality is an important and measurable dimension of NP. We sampled participants with different occupations and durations of NP but found no suggestion that these factors contributed to the differences in NP. Rather, NP was exacerbated by factors related to posture, activity or stress. In other cases, participants were unable to specify what aggravated their NP. This complexity suggests a multifactorial etiology. This complexity may partially explain the findings of systematic reviews that indicate most interventions for NP have small to moderate effects [26-28] and that within clinical trials, incomplete pain resolution is achieved [29]. One can assume that different presentations of neck pain might respond to different types of intervention or respond differently to the same intervention, thereby increasing the variability of observed treatment effects between and within trials.
Factors that exacerbated pain in the study were consistent with associations in published literature. A systematic review found that despite weak evidence on etiology, there was sufficient evidence that carrying loads can provoke low back pain, and trigger neck, thoracic, and shoulder pain [30]. This suggests that upper extremity function should be considered as salient items for neck disability outcome measures. Similarly, the fact that participants with chronic neck pain reported headache and neurologic symptoms in this study is in concordance with a previous qualitative study that investigated coping after whiplash where participants reported being challenged by neck and head pain, sensory hypersensitivity, and cognitive dysfunction [31].
The descriptions of typical pain with overlaid episodic exacerbations are consistent with epidemiological evidence that NP tends to be episodic. This study extended our understanding to include episodes that can be quite different across individuals. In some individuals, episodes of increased pain were predictable, whereas, in others, they were highly variable in terms of the nature, intensity, and predictability. Participants usually indicated that the interference in life was more related to the nature of the “hills” of pain exacerbations than the “valleys” of persistent pain intensity. This suggests that over time people learn to cope with the usual moderate symptoms, but are challenged when exacerbations arise.
Participants in the study acknowledged that stress exacerbated their NP. This appears contradictory to a qualitative study where family physicians reported that patients with NP have difficulty in accepting psychological explanations [32]. It might be postulated that patients are more able to accept stress as a mediator of their pain than as the “cause”, since the latter might imply to patients that people think it is “not real”. This suggests that health care providers should approach patients about stress management as a means of reducing their NP symptoms rather than focusing on causation in an attempt to promote a more successful therapeutic relationship.
Respondents in this study indicated that some types of pain were more problematic than others. For example, burning pain was rated as more intense both in interviews and the quantitative ratings. We hypothesize that when respondents report “burning” pain, it reflects neuropathic symptoms. However, our study did not include diagnostic tests that would have confirmed this. Neuropathic pain may be a distinctive subgroup of NP as suggested by a large epidemiological study where a neuropathic typology more easily differentiated pain subgroups then did medical diagnoses [33]. This suggests that a qualitative classification of pain could be an efficient and low-cost means to differentiate subtypes of NP. This approach has been used in neuropathic pain where diagnosis by self-report can be based on the Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) [34, 35]. If pain quality can differentiate subgroups that need different treatment approaches, then including pain subtype in future NP treatment algorithms or clinical practice guidelines might improve outcomes. For example, it might be possible to use diagnostic questionnaires like the S-LANSS [35, 36] to triage patients into different care pathways. Another approach to incorporating qualitative features into clinical decision-making would be to use the iconic measure used in this study [21-25]. Respondents in this study found the tool simple to use and there were consistent findings between the interviews and iconic ratings, suggesting that the information collected through the iconic tool was valid. This tool has undergone further refinement and is available for free use at https://app.painquilt.com/.
This study validated some aspects of the “axes” approach of pattern of NP that was used by the Neck Pain Task Force. Since few neck disorders can be identified through specific diagnostic tests such as imaging, the approach of using different classification axes might improve future research reporting. There are potential advantages and disadvantages to using a classification scale versus the open icon format. A classification scale may be useful for research subgrouping or prognosis in individual patients. The open iconic tool provides richer data that may enhance clinical interactions. Both formats need further investigation to determine utility.
The axes that The Neck Pain Task Force described for the nature of NP included severity, duration, and pattern. The classification characterized severity as a combination of symptom/pathology with disability on a four-point scale. This study suggests this might be problematic since these two factors were not always linked and combining them may nullify the ordinal ranking. In our revised descriptive classification, we separated symptoms and disability into two axes for clearer classification or case definition. Future studies validating this approach may link standardized self-report measures of symptoms and disability to these subgroups—this would enable clinicians who are using validated evaluative scales to classify their patients with NP in a more consistent manner.
The Neck Pain Task Force classified the pattern of NP as “single episode, recurrent or persistent” [14]. Participants with persistent symptoms in this study exhibited two distinct subgroups. One group had predictable exacerbations associated with changes in activity or stress. They often indicated that they made decisions about increased activity knowing they would “pay for it”. The other subgroup experienced unpredictable and bothersome episodes that were not clearly linked to any particular activity, time or stress. Respondents who had these latter types of exacerbations found them distressing and difficult to manage which suggested it was worth recognizing this distinction. Thus, we separated persistent - stable, and persistent - unstable subgroups.
Finally, The Neck Pain Task Force did not consider the location/distribution of NP symptoms in the classification system. By mapping symptoms both verbally and with icons on anatomic diagrams, we were able to identify that some participants present with localized NP, whereas others have very diffuse symptoms, suggesting that anatomical distribution is an important consideration. Further, participants with larger anatomic distributions tended to express more pronounced interference or bothersomeness. Thus, we proposed an ordinal measure of involvement for anatomical distribution as a component (or axis) of a descriptive classification.
We have compiled our findings into a proposed descriptive classification system that includes seven components or “axes”. It bears some similarity to the one proposed by The Neck Pain Task Force, including how the source and sampling are defined. However, it extends the descriptive component of the classification system to provide more distinct and comprehensive sub-typing. Neither classification has been empirically validated. Classification systems should be tested for their clinical utility in prospective cohorts to determine whether they can differentiate prognostic subgroups or identify those who would respond to different treatment approaches. Only once this validation has occurred should they be included in clinical decision rules for treatment selection or practice guidelines.
We attempted to ensure validity of our findings by using triangulation of data sources. Both member checking and comparison of qualitative and quantitative data supported the validity of our findings. However, we recognize there are limitations in our methodology and our sample that affect generalizability. Our sampling resulted in participants that were primarily university-educated, with a history of persistent NP. In addition, only 1 male participant was interviewed. Although NP is more common in females, we cannot be confident that our results extend to the broader population of people with NP, particularly those with acute NP or injury. Although we integrated our findings with existing literature, the validity of our descriptive findings does not necessarily mean that the classification system we derived from them is also valid. Although we suggest that pain outcome measures might be enhanced with measurement of pain quality, head-to-head psychometric evaluation of numeric pain rating and pain outcome measures that include items addressing the nature of pain is needed to determine whether these tools are superior.
Our findings confirm that NP is a complex experience and suggests that existing descriptive classifications may not be sufficiently comprehensive. It also suggests that measuring pain intensity alone will provide a very limited view of what the person with neck pain is experiencing. A descriptive structured pain scale was useful to describe the NP in terms of pain quality, location, and intensity. Based on the dimensions that emerged from our qualitative findings, we suggest there is value in using a seven-axis classification and a more comprehensive pain scale in clinical studies of neck pain and in practice. The checklist can be used to describe/classify the following components of NP: the source/ context, subgroup, distribution of symptoms, duration, episode/pattern, pain/symptom severity, and disability/participa-tion restriction. Further studies are needed to determine if the classification system or any of its components are useful in differentiating clinically meaningful subgroups of NP. Future clinical trials should incorporate standardized classification of the types of NP for participants recruited into studies as a means of facilitating these investigations.
CONCLUSION
Chronic components and acute exacerbations of neck pain vary substantially across individuals and over time. Multiple dimensions of pain contribute across a series of dimensions that include seven axes: source/context, sample subgroup, distribution, duration, episode pattern, pain/symptom severity, disability/participation restriction. Outcome measures and neck pain classification should consider the complexity of NP. Quantitative evaluation of proposed classification should be performed before implementation.
LIST OF ABBREVIATIONS
| NP | = Neck Pain |
| S-LANSS | = Self-completed Leeds Assessment of Neuropathic Symptoms and Signs |
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the Canadian Institutes of Health Research (CIHR) grants FRN: 102084, 114380, 117420. Joy C MacDermid was supported by a CIHR Chair in Gender, Work and Health and the Dr. James Roth Research Chair in Musculoskeletal Measurement and Knowledge Translation.
Additional acknowledgements are provided to Liora Bliumkin and Janice Cheung for assistance in conducting the interviews.


