RESEARCH ARTICLE
Effects of Modification of Pain Protocol on Incidence of Post Operative Nausea and Vomiting
Ran Schwarzkopf 1, *, Nimrod Snir2, Zachary T. Sharfman2, Joseph B. Rinehart3, Michael-David Calderon3, Esther Bahn3, Brian Harrington3, Kyle Ahn3
Article Information
Identifiers and Pagination:
Year: 2016Volume: 10
First Page: 505
Last Page: 511
Publisher ID: TOORTHJ-10-505
DOI: 10.2174/1874325001610010505
Article History:
Received Date: 25/07/2016Revision Received Date: 29/09/2016
Acceptance Date: 05/10/2016
Electronic publication date: 31/10/2016
Collection year: 2016

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Background:
A Perioperative Surgical Home (PSH) care model applies a standardized multidisciplinary approach to patient care using evidence-based medicine to modify and improve protocols. Analysis of patient outcome measures, such as postoperative nausea and vomiting (PONV), allows for refinement of existing protocols to improve patient care. We aim to compare the incidence of PONV in patients who underwent primary total joint arthroplasty before and after modification of our PSH pain protocol.
Methods:
All total joint replacement PSH (TJR-PSH) patients who underwent primary THA (n=149) or TKA (n=212) in the study period were included. The modified protocol added a single dose of intravenous (IV) ketorolac given in the operating room and oxycodone immediate release orally instead of IV Hydromorphone in the Post Anesthesia Care Unit (PACU). The outcomes were (1) incidence of PONV and (2) average pain score in the PACU. We also examined the effect of primary anesthetic (spinal vs. GA) on these outcomes. The groups were compared using chi-square tests of proportions.
Results:
The incidence of post-operative nausea in the PACU decreased significantly with the modified protocol (27.4% vs. 38.1%, p=0.0442). There was no difference in PONV based on choice of anesthetic or procedure. Average PACU pain scores did not differ significantly between the two protocols.
Conclusion:
Simple modifications to TJR-PSH multimodal pain management protocol, with decrease in IV narcotic use, resulted in a lower incidence of postoperative nausea, without compromising average PACU pain scores. This report demonstrates the need for continuous monitoring of PSH pathways and implementation of revisions as needed.
INTRODUCTION
Postoperative nausea and vomiting (PONV) is a common and disturbing problem for patients. In the first 24 hours after surgery the rates of nausea and vomiting are approximately 52% and 25% respectively [1]. Furthermore the risk of nausea after joint replacement surgery is 61% with 42% vomiting, as found by Koivuranta et al. [1]. Many risk factors predisposing to PONV in adults have been studied and in the high risk population, 79% of these patients may experience PONV [2]. Risk factors for PONV include: female sex, non-smokers, younger age, general anesthesia, volatile anesthetics and nitrous oxide, and duration of anesthetic [2, 3]. Postoperative opioids were also found as an individual risk factor for PONV [2, 3].
In addition to the high incidence of PONV and the distress it causes to patients, PONV is associated with economic implications for overall health care costs. Patients with unresolved PONV may require extended stays in the postoperative anesthesia care unit (PACU), increased total length of stay [4] and increased burden on the health care system [5, 6]. By reducing PONV, patients are able to mobilize earlier and facilitate an early active rehabilitation protocol [7]. Enacting measures to reduce PONV was shown to significantly reduce associated health care costs while improving quality of care for the patients [4, 7, 8]. Recently, the use of peri-articular local anesthetic (PAI) blocks in total joint arthroplasty has increased. PAI techniques are simple to perform, and are associated with lower incidence of PONV outcomes when compared to general anesthetic [8] or postoperative epidural analgesia alone [9].
Using a Perioperative Surgical Home (PSH) care model, patient care is standardized throughout the perioperative course. This study is aimed to compare the incidence of PONV in patients who underwent primary total knee arthroplasty (TKA) and total hip arthroplasty (THA) before and after modification of our PSH based multimodal pain protocol. We hypothesized that substituting IV opioid with other medications in the PACU would lead to a decrease in PONV without an increase in reported pain scores.
MATERIALS AND METHODS
The institutional review board approved this study. We conducted a retrospective chart review of prospectively gathered data. All primary Total Joint Replacement (THA and TKA) patients between October 2012 and December 2014 were included. A total of 149 primary total hip arthroplasty (THA) procedures and 212 primary total knee arthroplasty (TKA) procedures were included.
A standard multimodal pain protocol was studied from October 2012 to April 2014 and is presented in Fig. (1). Subsequently, the modified multimodal pain protocol was implemented in May 2014 with the purpose of reducing the incidence of PONV in this population (Fig. 1). The changes in the protocol included an additional single dose of IV ketorolac (15mg) given at the end of surgery in the Operating Room (OR) and postoperative oxycodone (immediate release 5-10mg orally) as needed for pain control instead of IV hydromorphone in the Postoperative Anesthesia Care Unit (PACU) (Fig. 1).
The primary outcome was incidence of post-operative nausea and vomiting in the PACU and was measured by the PACU nurse in hourly intervals as long as the patients were treated in the PACU. Secondary outcomes were post-operative average pain scores in the PACU, which were measured with a 10cm Visual Analog Scale (VAS) for pain recorded by the PACU nurse at the same time intervals as PONV assessment. Additionally, this study examined the effect of primary anesthetic modality (spinal vs. general anesthesia) on PONV and VAS pain score outcomes.
Statistical Analysis
Scalar variables are reported as mean ± standard deviation and were compared using t-test. Categorical variables were compared using χ2. Significance level was set at 0.05 and confidence intervals (CI) are reported at the 95th percentile. IBM® SPSS® 21 for windows was used for all analyses (IBM, Armonk, NY, USA).
RESULTS
There were no significant differences between the study groups regarding age, BMI gender or ASA classification (Table 1). The incidence of nausea in the PACU decreased significantly in the patients who received the modified protocol (27.4%) compared to the original protocol cohort (38.1%), (p=0.0442, CI of difference -20.8% to -0.6%). The rate of vomiting was 15.4% under the original protocol, and 14.3% under the modified protocol, a non-statistically significant difference (p=0.371). There was no difference in the rates of nausea or vomiting based on choice of anesthetic (spinal vs. general, p=0.67) or arthroplasty procedure performed (TKA vs. THA p=0.76). Fig. (2) shows nausea rates in each procedure group before and after the protocol change. Average PACU pain scores were lower with the modified protocol when compared to the original protocol but the difference was not statistically significant (1.7 ±2.6 and 2.0 ±2.4 respectively p=0.15) (Fig 3).
 |
Fig. (1). Total joint replacement perioperative surgical home multimodel pain management protocol. |
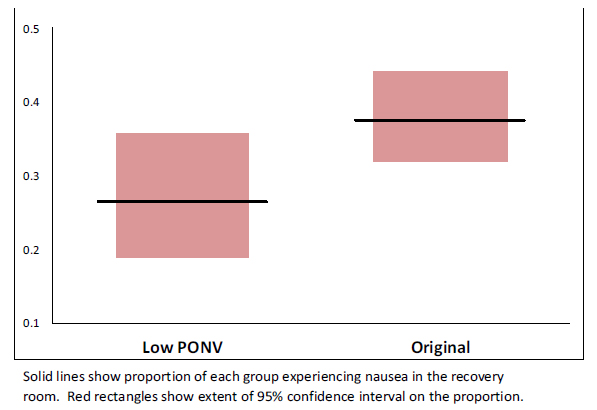 |
Fig. (2). Proportion of patients with nausea by group. |
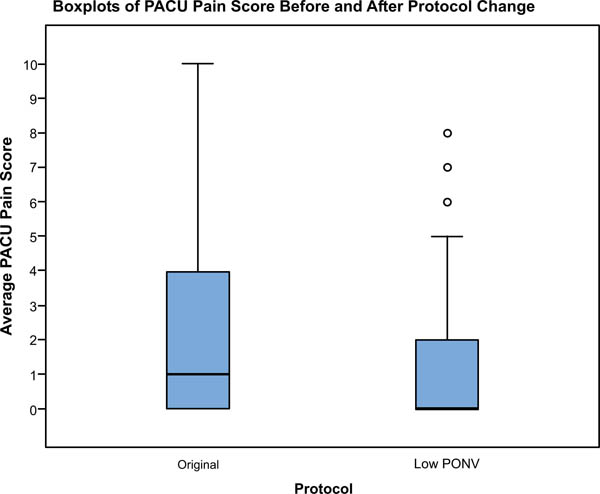 |
Fig. (3). Boxplots of PACU pain score before and after protocol change. |
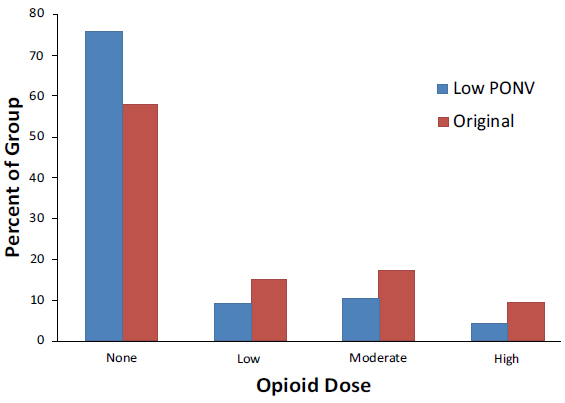 |
Fig. (4). Recovery room opioid dose by group. |
| Modified | Original | p | ||
| n=117 | n=245 | |||
| Age | 64.1 ± 12.8 | 63.5 ± 14.1 | 0.69 | |
| BMI | 29.2 ± 6.2 | 29.6 ± 6.4 | 0.58 | |
| Gender (M) | 45% | 36% | 0.11 | |
| ASA | ||||
| I | 0% | 0% | 0.211 | |
| II | 27% | 19% | ||
| III | 72% | 75% | ||
| IV | 1% | 5% | ||
Recovery room opioid use was classified as no use, low use (1 administration), moderate use (2-3 administrations) and high use (4+ administrations). The modified protocol group had a higher percent of patients who did not require any PACU opioids and fewer patients who used low, moderate and high dose opioids, this result did not reach significance (Fig 4).
DISCUSSION
The primary outcome of this study reports decreased incidence of postoperative nausea without changes in PACU VAS pain scores. This effect was observed after simple modifications to our total joint replacement PSH multimodal pain management protocol. The modifications entailed adding 15mg IV Ketorolac at the end of surgery and replacing IV Hydromorphone in the PACU with oral immediate release Oxycodone (5-10mg).
IV narcotic use is known to be associated with increased PONV. The decreased postoperative nausea observed in this study may be partially due to replacing PACU hydromorphone with oxycodone. The benefits of decreased postoperative nausea must be weighted against a possible increase in postoperative patient reported pain scores. In this study there was no associated increase in postoperative VAS pain scores with a decrease in morphine consumption. Based on these results the TJR-PSH modified protocol proved superior to the original protocol.
PONV remains of concern after TJR as it may predispose to decreased mobility, aspiration, electrolyte imbalances, thromboembolic disease, emotional distress, discomfort, and longer hospital stays [10]. Many antiemetic interventions to prevent PONV were shown to be similarly effective [8]. However, it is preferable to avoid nausea and vomiting than to treat them after presentation. Thus, the safest and least expensive prophylaxis should be utilized first [8]. In our modified TJR-PSH protocol this principal is employed by removing a high risk drug (IV hydromorphone) in an attempt to utilize fewer narcotic medications while maintaining adequate postoperative pain control and reducing PONV.
Ketorolac is a non-selective COX inhibitor indicated for acute moderately severe pain management. By decreasing formation of prostaglandin precursors ketorolac is known to have an antipyretic, analgesic and anti-inflammatory effect. The recommended dose of ketorolac is 120 mg per day or less for 5 days or less [11]. Contraindications to use are the same as for other NSAIDs. NSAID drugs are often associated with both gastrointestinal bleeding and operative sight bleeding. However, Storm et al. [12] found that the overall associations between ketorolac use and gastrointestinal bleeding and operative site bleeding are small. Physicians should however limit the dose and length of ketorolac use in older subjects.
Many total joint arthroplasty protocols use Ketorolac postoperatively in the ward or as part of the peri-articular injection cocktail [13-16]. Parvtaneni et al. [13] used peri-articular injections with a multimodal protocol that employed postoperative ketorolac and found excellent pain control and functional recovery compared to conventional pain control modalities. Pagnano et al. [14] has used Ketorolac for breakthrough pain postoperatively for patients with VAS scores greater or equal to 4. The modified protocol used in this study takes advantage of the known benefits of ketorolac in the PAI cocktail and postoperative use. Additionally, the modified protocol also uses IV ketorolac at the end of surgery in the operating room and takes advantage of the postoperative analgesic and antipyretic properties of the drug [17, 18].
A limitation of this study includes the retrospective nature of the research. Furthermore, this protocol can only be used in patients without contraindications to ketorolac. The two protocols were not studied simultaneously and the modifications made to the original protocol were not made in a stepwise fashion. Therefore it was not possible to measure the individual effects of adding postoperative IV ketorolac or removing hydromorphone. Although the study is well powered to analyze the primary outcomes, the study is underpowered to study the possible complications of ketorolac, none of the patients in the study had any reported complications from ketorolac. However, the use of ketorolac is indicated in this population group and recommendations for dosing and length of use were not violated.
CONCLUSION
This study reports how an additional single dose of IV ketorolac and postoperative oxycodone instead of IV hydromorphone in the PACU resulted in decreased incidence of postoperative nausea without worsening average PACU pain scores. This report demonstrates the need for continuous monitoring of PSH pathways and implementation of revisions as needed.
LIST OF ABBREVIATIONS
| ASA | = Anesthesia score assessment |
| BMI | = Body mass index |
| PACU | = Post acute care unit |
| PONV | = Post operative nausea and vomiting |
| PSH | = Perioperative surgical home |
| TJR | = Total joint replacement |
| VAS | = Visual analog scale |
CONFLICT OF INTEREST
RS- Smith & nephew, Intelijoint, Gauss Surgical.
ACKNOWLEDGEMENTS
Declared none.








