RESEARCH ARTICLE
Are We Economically Efficient Enough to Increase the Potential of in Vitro Proliferation of Osteoblasts by Means of Pharmacochemical Agents?
Mehmet Isyar1, Seyit Ali Gumustas2, Ibrahim Yilmaz3, *, Duygu Yasar Sirin4, Hacı Bayram Tosun5, Mahir Mahirogullari1
Article Information
Identifiers and Pagination:
Year: 2016Volume: 10
First Page: 420
Last Page: 430
Publisher ID: TOORTHJ-10-420
DOI: 10.2174/1874325001610010420
Article History:
Received Date: 05/02/2016Revision Received Date: 16/03/2016
Acceptance Date: 19/06/2016
Electronic publication date: 09/09/2016
Collection year: 2016

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Background:
The aim of this study was to test the necessity of using expensive and unaccesible pharmacological-chemical agents in the proliferation of bone tissue cultures and in the induction of mineralized matrix formation to increase the osteogenic effect.
Methods:
For this purpose, human primary cell cultures were prepared and then divided into two groups. Whereas the cells in group I were fed with an osteoblast stimulator medium containing Dulbecco’s Modified Eagle Medium (DMEM) and β-glycerophosphate, the cells in group II were fed with DMEM containing dexamethasone and 2-phospho-L-ascorbic acid trisodium salt. Both groups were evaluated in terms of viability, toxicity, and proliferation and then compared in terms of cell surface morphology through inverted light and environmental scanning electron microscopy. In addition to immunoflow cytometric analyses, the effects of alkaline phosphatase activities were evaluated using the spectrophotometric method to examine the osteoblastic activities. Costs were calculated in the currency of the European Union (Euros). The Tukey Honestly Significant Difference test was used to reach the statistical evaluation of the data after the analysis of variance.
Results:
It was reported that the level of the alkaline phosphates was higher in group I compared to group II. It was observed that the surface morphology quality, the number of living cells, and proliferation were higher in group II and that the results were deemed statistically significant.
Conclusion:
It was found that the 2-phospho-L-ascorbic acid trisodium salt and dexamethasone mixture was as effective as the expensive commercial kits on the osteogenic effect on human primary bone tissue.
1. INTRODUCTION
Globally, research is being focused on obtaining regenerative cells cultured in laboratories to obtain a repeatable therapy procedure for the treatment of incurable diseases [1, 2]. In order to repair damaged cells and tissues and to replace the biological activities of cells, cell culture techniques have been used for cell treatment which is one of the research objectives of orthopedic surgery [3, 4].
It is well-reported in the literature that the cells generated in laboratories behave distinctly in accordance with the environment of their culture conditions and differentiate in accordance with the stimulants used as pharmacological-chemical agents [3-5]. To sustain the cell differentiation, increase viability and proliferation, and preserve and maintain cells, different mediums and a wide range of ingredients such as amino acids, carbohydrates, lipids, and vitamins have been developed in recent years [5-7]. With the current increase in the number of for-profit health organizations, cost calculations have become a major consideration. Today, health economics has come to the forefront, and there has been an effort to lobby for the more efficient use of limited resources and to take precautions against rising costs. Research proposing decreased costs have been supported [8]. It is an important goal to determine the most efficient methods to reduce the economic burden of molecular-based experiments. Some researchers have emphasized that it is possible to realize cell proliferation with inexpensive equipment and agents [9, 10].
From this perspective, we have studied three different pharmacological-chemical agents to test the hypothesis that inexpensive and easily accessible substances may be applied, instead of expensive and more difficult to obtain substances, to increase the osteogenic effects and mineralized matrix formation in primary cell cultures isolated from human bone tissue.
2. MATERIALS AND METHODS
This study was conducted with the permission of the Local Ethics Evaluation Commission of the Istanbul Medipol University School of Medicine, Istanbul, Turkey. The signed informed consent forms were obtained from the participants giving permission to conduct research on the tissues.
2.1. Materials
Collagenase Type I enzyme (1 mg/mL; Invitrogen Corporation, Carlsbad, CA, USA), Hank’s Balanced Salt Solution (HBSS-1X, catalog #14025, Gibco, penicillin-streptomycin (PS), fetal bovine serum (FBS), L-glutamine (200 mM), and Dulbecco’s Modified Eagle’s Medium (DMEM 1000 mg glucose/L) were obtained from the Sigma-Aldrich Corporation, St. Louis, MO, USA. The MesenCult Osteogenic Stimulatory Kit for MesenCult-XF (catalog #05434, containing MesenCult Basal Medium, catalog #05431), Osteogenic Stimulatory Supplement (catalog #05435), and β-glycerophosphate (catalog #05436) were obtained from STEMCELL Technologies, Vancouver, Canada.
The osteogenic media supplements including dexamethasone (catalog #D4902-50-02-2), 2-phospho-L-ascorbic acid trisodium salt (PAAT) (49752-100G), and p-nitrophenyl-phosphate substrate (N7653), used as a coloring agent in the measurement of alkaline phosphatase (ALP; IU/L) activities, were obtained from the Sigma-Aldrich Corporation, St. Louis, MO, USA.
Cell culture supplies were bought from Sarsted and Greiner. MTT assays were performed using the Vybrant MTT Cell Proliferation assay (catalog #V-13154) obtained from Cell Biolabs Inc., San Diego, CA, USA. The laminar flow cabinet (Air Flow-NUVE/NF–800 R) and incubator (NUVE, 06750) were obtained from Ankara, Turkey. An Olympus CKX41 inverted microscope was used for light microscopy, in conjunction with the Olympus Cell Soft Imaging System software. A Mindray MR-96A (Mindray Medical International Limited, Shenzen, China) enzyme linked immunosorbent assay (ELISA) was used for cell viability and cytotoxicity measurements.
A Quanta 250 FEG (FEI Company, Hillsboro, Oregon, USA) environmental scanning electron microscope (ESEM) was used for electron microscopy.
2.2. Methods
2.2.1. Eligibility Criteria
Cases with Paget disease, primary/secondary hypo/hyper-parathyroidism, micro osteoporosis, acromegaly, primary osteogenic sarcoma, and bone metastasis were not included in the study. Also, the cases that used drugs that would interact with cytochrome p-450 enzyme (CYP2A6) or disease-modifying anti-rheumatics such as rituximab, etanercept, adalimumab, and abatacept were not included in the study [11].
2.2.2. Preparation of Primary Osteoblast Culture
Primary cell cultures were prepared from leftover bone tissues from cases (n = 6) who underwent arthroplastic knee surgery (Fig. 1). Cases were graded by the Kellgren-Lawrence grading scale [12], and those not responding to medical and conservative treatments who had large osteophytes and were an average of 69 years of age were selected.
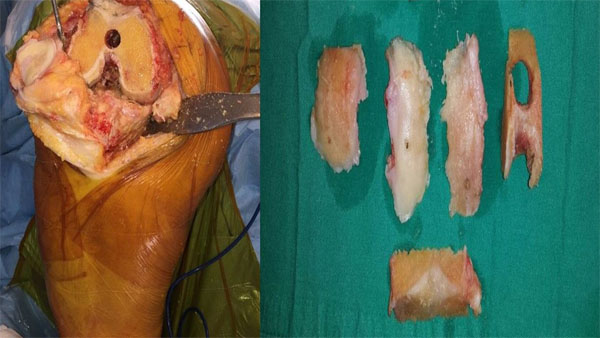 |
Fig. (1). Removal of bone tissue during total knee prosthesis. |
Tissues were transferred to the laboratory at 4°C in a DMEM medium containing 5% PS. In a laminar flow cabinet, samples were washed with sterile phosphate buffered saline (PBS) in order to purify the blood, and rinsed. Next, they were cut into small pieces with a rongeur and surgical blades. Samples were then washed in a 50 mL 0.1% (w/v) HBSS solution. To perform enzymatic digestion, a 200 units/mL collagenase type II enzyme mixture was dissolved in complete medium. Next, the tissue samples were incubated for 16 h in a CO2 incubator. Afterwards, tissue samples were centrifuged at 4°C at 1,200 rpm for 10 min to discard collagenase. Sedimented bone tissue pellets were resuspended in a fresh culture medium (DMEM) and transferred to flasks to obtain primary cultures. The complete culture medium (FBS, PS, and DMEM) was changed every two days. The human primary osteoblast cell cultures that reached approximately 90% confluence were used for the experiments.
Afterwards, cells were detached from the flasks with trypsin and counted using a Thoma cell counting chamber in the presence of trypan blue. The 1.6x105 osteocytes were put into six-well plates and supplemented with different mediums as follows: Group I–DMEM containing 5 mM osteoblast stimulator kit (OSK) and Group II–DMEM containing 50 μg/mL of PAAT and dexamethasone (10-8 M).
The cells in group I were fed by means of an osteoblast stimulator kit containing DMEM and β-glycerophosphate (175-μL) [(47.5-mL MSC Basal Medyum) + (2.5-mL Osteogenic Stimulator Supplement) (OSK)]. On the other hand, the cells in group II were fed with a DMEM 50 μg/mL PAAT and dexamethasone (10-8 M) supplement [13]. Until the second passage, the cells were fed with different cell media every two days (Table 1).
MTT-ELISA cell viability, toxicity, and proliferation tests, ESEM analyses, immunoflow cytometric analyses, and alkaline phosphatase enzyme activity analyses were carried out on days 1, 7, 14, and 21 in both groups.
| Groups | Medium and additives | Osteogenic stimulatory medium |
|---|---|---|
| Group I | DMEM, FBS, PS | Only OSK |
| Group II | DMEM, FBS, PS | PAAT + Dexamethasone |
DMEM: Dulbecco’s Modified Eagle Medium; FBS: Fetal bovine serum; PS: Penicillin-streptomycin ; OSK: Osteoblast stimulator kit; PAAT: 2-phospho-l-ascorbic acid trisodium salt.
2.2.3. Analysis
2.2.3.1. Scanning Through Inverted Light Microscopy
Cell viability, morphology, and confluency were monitored with inverted light microscopy and micrographs obtained in 4x, 10x, 20x, and 40x magnifications.
2.2.3.2. Immunoflow Cytometric Analyses
Cells were detached with trypsin-EDTA (0.25%) and then centrifuged at 4°C three times for five min at 2,000 rpm and washed with fresh medium each time. The pellets were resuspended in a freshly prepared cell culture medium, transferred into falcon tubes, and incubated with fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated CD14, CD34, and CD44 monoclonal antibodies against cell surface markers, with appropriate controls at 4°C for 25 min, protected from the light. The cells were washed by the addition of PBS containing 0.1% sodium azide and then centrifuged for 5 min at 1,300 rpm at 4°C. The supernatant was removed, and the cells were resuspended in an assay buffer and analyzed with a flow cytometer. Results were evaluated using the BD FACSCalibur Cell Quest software (Becton Dickinson, Palo Alto, CA, USA) [11, 14, 15].
2.2.3.3. Osteogenic Differentiation of Cell Cultures
Alkaline phosphatase (ALP) activity is one of the most commonly used markers for osteogenesis since it reflects the proportion of osteogenically differentiated cells. ALP activity was determined using a standard curve obtained from p-nitrophenyl-phosphate substrate as a substrate. To study the osteoblastic differentiation, cells/mediums were collected and stored at -20°C on days 7, 14, and 21 after being put in well plates. The collecting environment was diluted with a solution containing four times of buffer solution (1mol/1Diethanolamine), (0.5mmol/L MCL2), and one time of substrate solution (10mmol/L p-nitrophenyl-phosphate substrate) [16].
2.2.3.4. ESEM Analyses
In order to assess the surface topography and composition of the cells, ESEM analysis was carried out. The culture medium was removed from the plates, and a 2.5% glutaraldehyde solution, composed of 97.5 mL of cacodylate buffer and 2.5 mL of glutaraldehyde, was added to the dish to cover the cells. The glutaraldehyde solution was then removed, and cells were maintained at room temperature for 2 h, prior to three washes using cacodylate buffer. After the last wash, the cells were covered with cacodylate buffer and stored at 4°C prior to analysis [14, 15, 17, 18]. Images of the extracellular matrix and characteristic cellular structures were obtained.
2.2.3.5. Determining Vitality and Toxicity Effects: MTT-ELISA Analyses
Vitality tests were performed using a commercial MTT kit, according to the manufacturer’s instructions. MTT assays were carried out on osteocytes at days 1, 7, 14, and 21 in both groups. The culture media was discarded from the wells by a gun pipette. Instead of the removed supernatant, 12 mM/5-mg MTT tetrazolium solution, 1 mL sterile PBS, and DMEM were added, followed by sodium dodecyl sulphate (SDS) prepared by adding 10 mL 0.01 M HCl to 1 g SDS) and then by a 100 μL MTT stock solution. Cultures were incubated at 37°C for 2 h and protected from the light. A 500 μL aliquot of the resultant reaction solution was removed for analysis. Dimethyl sulfoxide was added to these samples and then incubated at 37°C for an additional 10 min, prior to photometric analysis of a 540 nm wavelength absorbance.
The proliferation of the increased living cells in the culture was calculated in percentages using the following formula: proliferation = Test (OD/Control OD) × 100. On day 1, the number of living cells was regarded as 100%. Afterwards, the number of cells was reported in percentages on days 1, 7, 14, and 21. The difference in proliferation was investigated [3, 14, 15, 17, 19].
2.2.3.6. Cost Analysis
In order to obtain condensation, differentiation, and healthy proliferation, the cost of pharmacological and chemical agents was calculated by multiplying the number of units by unit cost obtained from the suppliers’ price lists (10). The costs were calculated in euros (1 Euro = 3.132 Turkish Liras).
2.2.3.7. Statistical Analysis
The results were evaluated through cell proliferation in the data analyses. The results were expressed as mean ± standard deviation. The data input of costs was done using Microsoft Office Excel 2010. The results were presented in percentages. Statistical evaluations were carried out via the Minitab 16 (Minitab Inc., State College, PA, USA) program. Comparison analyses were scored by means of variance analysis (ANOVA). The Tukey Honestly Significant Difference (HSD) test was used to assess whether the significant input between groups was important or not. The alfa significance value p was < 0.05.
3. RESULTS
3.1. Evaluation of Inverted Light Microscopy
It was reported that the cells were healthy and proliferous before the experiment and passage (phase 2) process (Fig. 2). In the culture samples in group II, the images where the confluent rates were 90% (15/16) were reported.
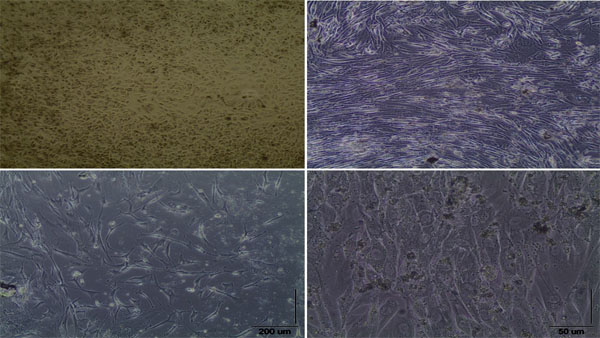 |
Fig. (2). Inverted light microscopy image. |
3.2. Evaluation of Immunoflow Cytometric Analyses
Immunoflow cytometric analyses of CD14, CD34, and CD44 monoclonal antibodies concluded that the cells stuck at the bottom were osteocytes with activities (Fig. 3). Whereas the samples from CD14 and CD34 had negative expressions, CD44 had a positive expression (99.08%), which showed that it was more than in group II.
 |
Fig. (3). Evaluation of immunoflow cytometric analyses. |
3.3. Evaluation of ALP Activity
These analyses indicated that the amount of alkaline phosphates in group II was far more than in group I (Fig. 4).
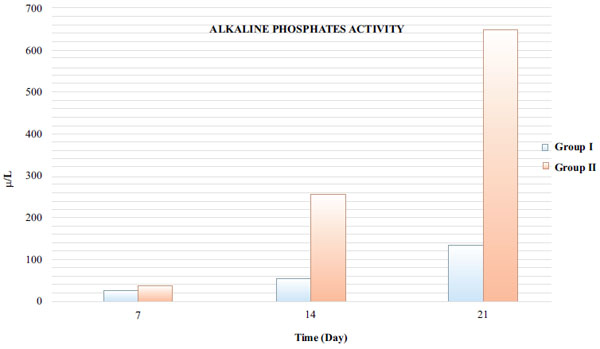 |
Fig. (4). The result of ALP tests on days 7, 14, and 21 in media. |
3.4. Evaluation of ESEM Analyses
The number and distribution of the cells in group II were far more regular and homogeneous when compared to group I. Photographs showed that the density of the matrix and its environmental extension increased significantly on day 21 (Fig. 5).
 |
Fig. (5). Characterization of cell surface morphology. |
In the images of the samples belonging to group I, it was shown that the cells’ extracellular matrix was destroyed, they had lost their glassy appearance, their concave shape was deformed, and they had left their isogons environment by splitting.
3.5. Statistical Evaluation of ELISA Data Obtained from Biochemical Analyses
In the evaluation of cell proliferation, it was found that the best osteoblastic activity, vitality, and proliferation were observed in group II when it was reported in percentages on days 1, 7, 14, and 21 (Fig. 6, Table 2).
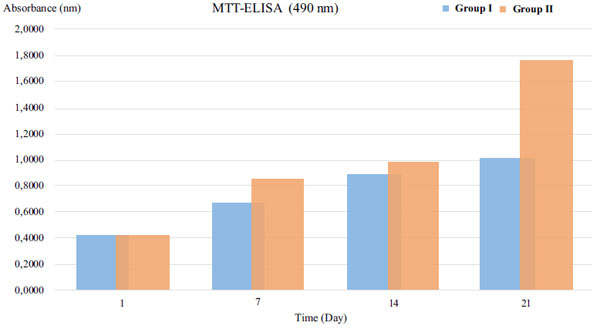 |
Fig. (6). Indication of cell vitality, toxicity, and proliferation. |
| Time (Day) | Group I | Group II | p* |
|---|---|---|---|
| 1 | 0.4120 g¥ | 0.4098 h¥ | 0.00 |
| 7 | 0.6650 f¥ | 0.8562 e¥ | 0.00 |
| 14 | 0.8920 d¥ | 0.9796 c¥ | 0.00 |
| 21 | 1.023 b¥ | 1.7750 a¥ | 0.00 |
*Analysis of variance test (ANOVA). ¥: The Tukey The Tukey Honestly Significant Difference (HSD) test.
3.6. Evaluation of Costs
The cost of the cell line for a passage was calculated when it was used solely or in combination with OSK, PAAT, and dexamethasone. When it was used solely or combined with OSK, it was found that the cost in euros for a passage was 78.04% and 71.79% higher, respectively.
4. DISCUSSION
In the literature, it is emphasized that the components of media for the culture of mammal cells should contain amino acids, vitamins, salt, glucose, antibiotics, and serum [3, 20]. It is also stated that the culture environment needs of the cells should be determined by the aim of the research. Moreover, it is emphasized that the media may differ by types of cells, adaptation capability, and types of cell source organisms so that cells maintain life and vitality [3, 20]. First and foremost, the necessity of using chemical and/or biological agents that may trigger the construction of intercellular webs in the reproduction and differentiation of cells is reported in nearly every study [3, 20-22]. It is known that molecular-based experiments where these agents are used represent a costly burden for health budgets. The goal was to find the most efficient method of conducting the experiments as well reducing the economic burden. The use of reasonably priced equipment has become essential in order to achieve these goals.
Laboratories where cell culture studies are conducted and live mammal subjects are used are quite expensive to establish and maintain. Also, equipment used in this type of research is expensive. Consumables and kits used in the evaluation of the data obtained from these experiments are expensive, too. In order to decrease the cost of monocular-based research, where cell culture studies are carried out in vitro, more economical solutions are needed. For this reason, we sought the possibility of using inexpensive chemicals to produce healthy cells. To do this, we used OSK and PAAT mixed with dexamethasone which is known to increase osteogenic effects. We applied OSK, which is expensive and difficult to obtain, in one group while we used a PAAT and dexamethasone mixture, which is inexpensive and readily available, in the other group. We compared them as to vitality, toxicity, proliferation, osteoblastic effects, and activities.
Jager et al. studied the effects of dexamethasone, ascorbic acid, and β-glycerophosphate in combination (DAG) and bone morphogenetic protein-2 (BMP-2) on osteoblastic modulation of mesenchymal stem cells in vitro, and they reported that BMP-2, when combined with DAG, had many more effects of osteoblastic modulation on mesenchymal stem cells [23]. Bone-based alkaline phosphatase, an isoform enzyme of alkaline, is a tetrameric glycoprotein existing on osteoblasts and getting into circulation by the enzyme phosphatide inositol-glycan. It joins the calcification of the bone matrix by pyrophosphate division which is a bone mineralization inhibitor. Therefore, bone alkaline phosphatide is known to be an accurate and definite indicator of bone metabolism. Peter et al. conducted an experiment on mice which showed that, when osteogenic yields such as dexamethasone, β--glycerophosphate, and L-ascorbic acid were added to a bone culture based on bone marrow, it significantly increased the production of ALP and osteocalcin, and it aided cell reproduction in osteoblastic phenotypes [24].
Chang al. studied the effects of glucocorticoids on the osteoblastic modulation of fetal and adult bone marrow. They reported the necessity of glucocorticoids for osteogenesis and showed that the potential for repair of fetal bone marrow cells was higher when compared to adult bone marrow cells [25].
Shima et al. carried out research in which they studied repair of bone tissue and remodeling. They added ascorbic acid, β-glycerophosphate, and dexamethasone, combined with a culture medium, in order to sustain differentiation in osteoblasts. As a result of their study, they indicated that the cells produced were able to be used in bone replacement [26].
In our study, we used a solution, the mixture of dexamethasone and PAAT, which is inexpensive and accessible. We observed that there were more osteoblastic modulation effects in the group with this solution than in the group in which OSK was used, and we concluded that there was a statistical significance.
De Fariave et al. characterized a convenient cell culture environment for osteoblast culture studies in vitro [27]. Cenni et al. studied the effects of active thrombocyte concentration on human fibroblast and osteoblast cultures. It was reported that there were no significant differences between the group which was treated with active thrombocytes in vitro and the group which was not treated with it in regard to osteoblastic and fibroblastic activities [28].
From the literature concerned with this subject, it may be concluded that animal tissues were generally evaluated. It is known the results may be misleading because human and animal tissues differ from each other [14, 15, 29]. Additionally, it becomes controversial in terms of the conformance of the in vitro tests because one type cell is used in cell line systems. Cells do not have complex coordination mechanisms with their micro environments, and cells are prevented from interacting with other formations such as extracellular matrix structures [14, 15, 29]. The main disadvantage of these cell lines is that they do not have the characteristics of their genotypic and phenotypic features because they are genetically modified cells [14, 15, 29, 30].
Cell line was excluded from the research, and human primary explant cell cultures were used instead of mesenchymal cell cultures. Although the study seems to be an in vitro experiment, the cell cultures were not derived from animal cells, and commercially prepared cell lines were not used. Because of these reasons, we are of the opinion that the results may contribute to the literature in terms of the abovementioned three points.
Miki et al. reported that the cell growth in the medium supplemented with aurintricarboxylic acid, which is much cheaper than IGF-1, combined with LPA was synergistically promoted similarly to that in the medium supplemented with IGF-1 and LPA. It can be concluded then that the serum-free medium, designed on the basis of general commercial media, could support the growth of CHO cells and antibody production comparable to the serum-containing medium in a suspension culture. Moreover, the possibility of the cost reduction by the substitution of IGF-1 with aurintricarboxylic acid was also shown [9].
In conclusion, as far as the cell proliferation is concerned, the best osteoblastic activity (CD44 positive expression 99.08%) was observed in group II on days 7, 14, and 21 in terms of vitality (p = 0.00). These analyses indicated that the amount of alkaline phosphates in group II was far more than in group I. ESEM screenings were parallel with MTT-ELISA analyses and the result of the alkaline phosphatase osteogenic activity test. These screenings, different from group I, showed that the samples in group II, in which PAAT and dexamethasone solution were added without OSK, had higher cell numbers and higher matrix density. Moreover, when OSK was combined or used alone, it was calculated to increase costs in euros by 78.04% and 71.79% per passage, respectively.
The study results showed that 2-phospho-L-ascorbic acid trisodium salt and dexamethasone have significant osteogenic effects on primary human bone derived cell culture, and further studies are recommended to evaluate these effects on the treatment of bone disorders.
CONCLUSION
This study is one of the few focusing on the cost calculation of stimulant agents in differentiation which are some of the most costly substances used in monocular research about stem cell cultivation. The results of this study showed that inexpensive and accessible kits may increase the osteogenic effects, which may contribute to the economic efficiency of the research.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.








