All published articles of this journal are available on ScienceDirect.
Methods and Guidelines for Venous Thromboembolism Prevention in Polytrauma Patients with Pelvic and Acetabular Fractures
Abstract
Sequential compression devices and chemical prophylaxis are the standard venous thromboembolism (VTE) prevention for trauma patients with acetabular and pelvic fractures. Current chemical pharmacological contemplates the use of heparins or fondaparinux. Other anticoagulants include coumarins and aspirin, however these oral agents can be challenging to administer and may need monitoring. When contraindications to anticoagulation in high-risk patients are present, prophylactic inferior vena cava filters can be an option to prevent pulmonary emboli. Unfortunately strong evidence about the most effective method, and the timing of their commencement, in patients with pelvic and acetabular fractures remains controversial.
INTRODUCTION
Venous thromboembolism (VTE) is a prevalent and severe disease compared with other public health problems [1-4]. The reported incidence of deep vein thrombosis (DVT) after pelvic fractures varies according to patient demographics, the type of fracture, and the method of detection [5, 6]. However, without thromboprophylaxis the incidence of DVT in these patients may be more than 50%, making prevention a crucial part of patient care [7, 8] (Fig. 1). Considering hospitalized patients, those who have suffered major trauma experience the greatest risk for this complication. In this group, when thromboprophylaxis was not administered, 40% to 80% had objectively documented deep vein thrombosis (DVT) [9-12]. It has been reported that approximately 80% of DVT are clinically silent and only 30% of fatal pulmonary embolism (PE) cases are detected prior to death [8]. In this major trauma group it has been published an incidence of death from PE of 0,4% to 2%, and often occurs without warning in the postoperative period, making it the most common source of morbidity and mortality in patients who outlast the first 24 hours and the most preventable cause of death in hospitals [13] (Fig. 2). A documented 61% incidence of DVT was identified following patients with pelvic fractures who had received no prophylaxis, and surprisingly, only 1.5% of the patients in this study with DVT had clinical characteristics evocative of thrombosis prior to the diagnosis on venography [3].
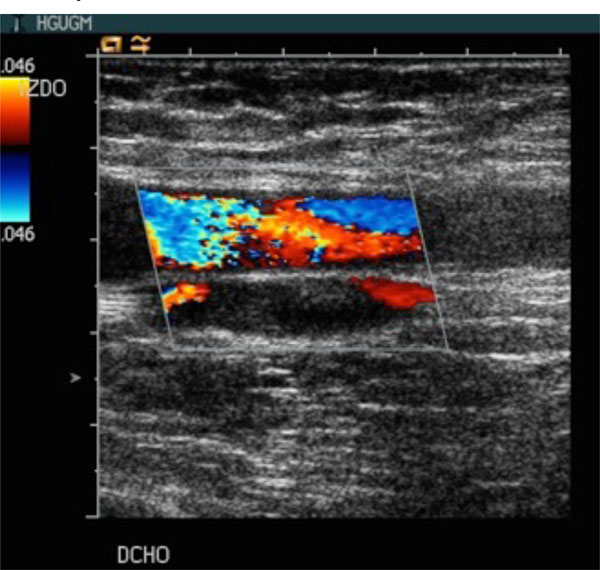
Color doppler ultrasound exam of a patient with acetabular fracture. Bilateral acute thrombosis of common femoral and deep femoral veins.
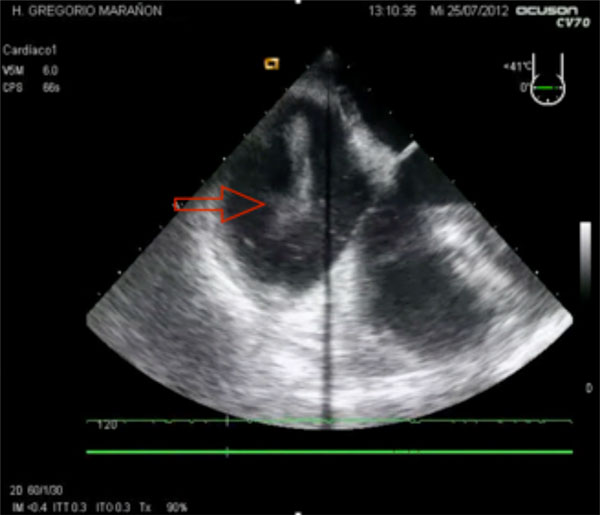
Echocardiography findings in a massive pulmonary embolism (PE), with free thrombus (red arrow) and marked dilation of the right ventricle.
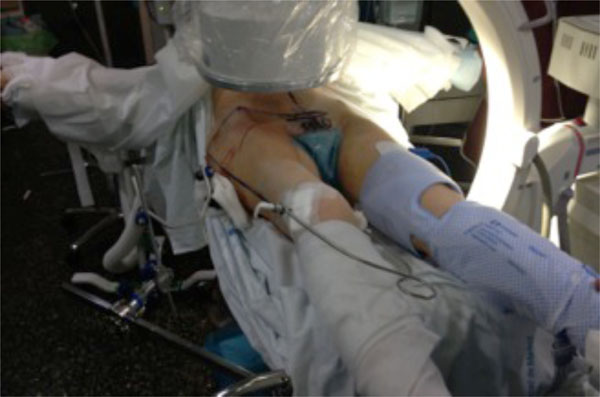
Intermittent compression device, used in conjunction with pharmacological thromboprophylaxis, during the surgical procedure.
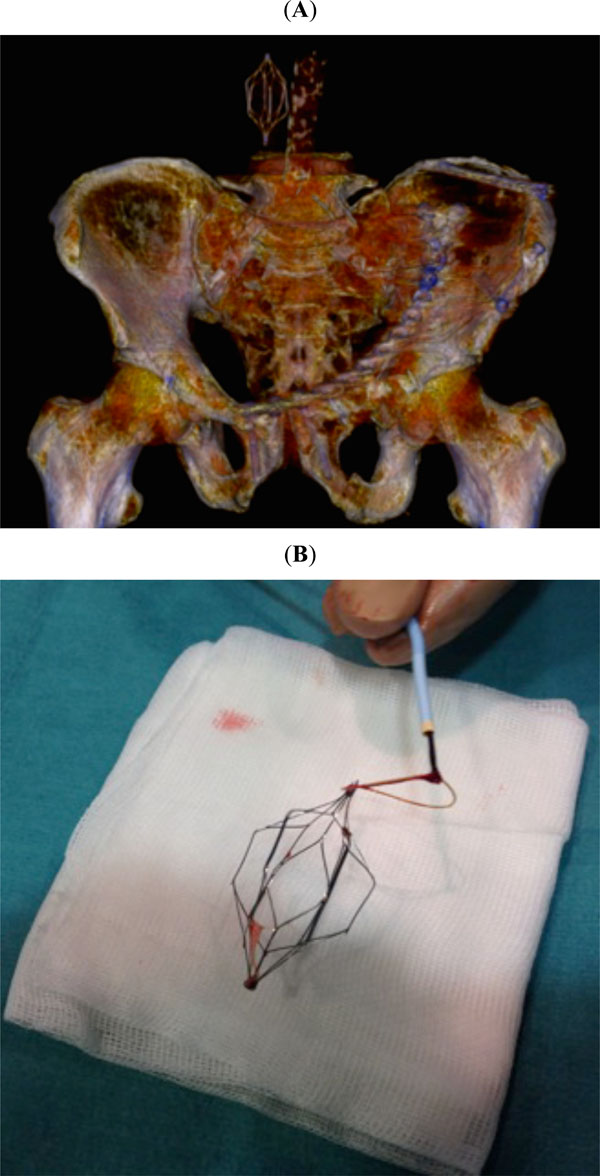
(A) Postoperative three-dimensional CT scan showing the inferior vena cava filter. (B) Retrievable filter is typically inserted and removed in a percutaneous way via the internal jugular or femoral veins.
Indications for insertion of IVC filter in trauma patients by the Eastern Association for the Surgery of Trauma [21].
| Traditional Indications (Level 1) | Extended Indications (Patient with Established DVT or PE) (Level 2) | Prophylactic Indications (Very High-Risk Trauma Patient) (Level 3) |
|---|---|---|
| Recurrent PE despite full anticoagulation Proximal DVT and contraindication to full anticoagulation Proximal DVT and major bleeding whilst on full anticoagulation Progression of iliofemoral clot despite anticoagulation | Large free-floating thrombus in the iliac vein or IVCFollowing massive PE in which recurrent emboli During/after surgical embolectomy | Chemical anticoagulation contraindicated due to increased risk of haemorrhage in the patient (e.g. intracranial haemorrhage, solid intraabdominal organ injury, coagulopathy) and patient with one or more of:- Severe closed head injury (GCS <8)- Incomplete spinal cord injury with para/quadriplegia- Complex pelvic fracture with associated long bone fracture- Multiple long bone fractures |
In patients using prophylaxis for DVT, the incidence is reported as low as 2% to as high as 33% [14]. The goal of all forms of DVT prophylaxis is to prevent long-term morbidity associated with DVT and ultimately the potential mortality associated with PE [15]. Several authors demonstrate that one in four PEs leads to mortality [16]. Hence, using thromboprophylaxis adequately is an essential step in the management of the patients who sustain a pelvic fracture [17].
In spite of representing a high-risk population for DVT, there are no current prophylaxis guidelines available and there is a lack of consensus, remaining of great interest the venous thromboembolism prevention for patients with pelvic or acetabular fractures.
This paper reviews the incidence, methods of thromboprophylaxis and guidelines available for prevention of VTE in pelvic trauma patients.
PATHOPHYSIOLOGY AND RISK FACTORS FOR VTE:
Pelvic and acetabular fractures usually result from high-energy trauma, associated with injury to vascular structures and oblige prolonged immobilization [17]. The classical triad of Virchow can explain the pathogenesis of venous thrombosis in these patients [10]. This triad labels the three factors that are thought to generate the thrombosis: hemodynamic changes, endothelial dysfunction and hipercoagulability status. In these cases the deficiency of the laminar flow through the venous system can be caused by immobilization due to traumatic radicular lesions, limb fractures, stabilization, anaesthesia or pain. This situation will weaken the calf pump and contribute to vascular stasis. Vascular endothelial dysfunction may result from direct trauma or by the use of surgical techniques that may promote tissue necrosis or direct vessel damage. Pelvic fractures are profound stimuli for the activation of the coagulation cascade. Although initially, in the acute phase, the significantly injured patient may develop a hypocoagulable status, once stabilized, trauma patients are disposed to suffer a state of hypercoagulability, being the most important factor in the development of acute DVT the imbalanced activation of the clotting cascade. Tissue factor and markers of thrombin generation increase after a major trauma, while the endogenous anticoagulants (i.e., antithrombin III) show a tendency to be decreased.
Most venous thrombi in high-risk patients proceed from the deep veins of the calf and stay subclinical if they do not spread proximally. 20% of calf thrombi do extend into the proximal veins. These proximal thrombi are significantly more severe, since at least 50% of these thrombi move to the lungs, causing a pulmonary embolism. In patients who undergo delayed open reduction and internal fixation these thrombi may be intensely relevant because intraoperative manipulation of the fragments and maneuvers with the inferior limbs may result in embolization from these thrombi [11]. A complication in the long term in these patients is the post-phlebitic syndrome. After a proximal DVT 25 to 50% of patients have chronic leg swelling, with pain, hyperpigmentation, and ulceration [18].
Risk factors for VTE after major trauma include increasing age, pelvic, spinal or head injury, lower limb fracture, prolonged immobility, ventilatory or haemodynamic instability and surgical procedures [4, 19]. When these factors are met, they have an accumulative effect in rising the risk of developing DVT [20]. Surprisingly, some authors suggest that there is no evidence that the risk of VTE correlates with the severity of the pelvic fracture [13].
The Eastern Association for the Surgery of Trauma (EAST) has published a guideline to state the level of evidence associated with each specific risk factor for DVT in trauma patients. The most convincing evidence for these authors is for patients with spinal fractures or spinal cord injuries. However, older age, higher injury severity score, need for blood transfusion, pelvic or long bone fractures, head or severe chest injuries and requirement for ventilatory support are considered with Level 2 evidence as risk factors for embolic complications in trauma patients [21].
Karunakar et al. heralded obesity as a risk factor for deep venous thrombosis [22]. Arroyo et al. defined additional characteristics influencing DVT following pelvic trauma, including respiratory disease, obesity, male sex, medical co-morbidities, and time to definitive surgery, being obesity and respiratory disease the most influential in the development of VTED [13]. In a trial of two hundred and thirty two patients, it has been described that a moratorium to surgery was associated with a significant increment in VTE [23]. While earlier intervention was associated with an elevation in mortality-risk, benefits such as reduced rates of VTED and infection can also be caused [24]. Another factor that should be taken into account is the surgical approach. Patients with posterior injuries operated via Kocher-Langenbeck approach, supported a significant higher risk [25].
As it is clear that the use of thromboprophylaxis in these patients is associated with a reduced frequency of DVT, some authors consider that identifying risk factors for individual pelvic fractures is not especially useful because all cases have a high risk of DVT, so modifying the prophylaxis for patients with different estimated risks would not reduce adverse outcomes [2, 13, 20]. On the other hand, it could be extremely useful to establish independent factors of VTE in patients who cannot use chemical prophylaxis in order to benefit from aggressive screening and prophylactic devices, as inferior vena cava filters (IVCF) placement. Malinoski, after logistic regression, found that past medical history of DVT (OR = 22.6) and any extremity fractures (OR = 2.4) remained as independent factors in critically injured patients who cannot use chemical prophylaxis and recommended aggressive screening and prophylactic IVCF placement when anticoagulation is prohibited [6].
THROMBOPROPHYLAXIS OPTIONS
The regular prophylaxis of VTE is part of daily critical care. As a general rule, the risk of DVT must be balanced against the complications associated with the use of these methods of thromboprophylaxis [16].
PHARMACOLOGICAL THROMBOPROPHYLAXIS
All these drugs interrupt the coagulation cascade targeting different steps, in order to inhibit the formation and extension of thrombi. On the other hand, with the use of chemical prophylaxis, the risk of bleeding is present. Several options warrant attention in pelvic surgery patients. Unfortunately there are few randomized trials specifically designed in patients with pelvic fractures. Thus, no consensus exists on the most effective protocol for DVT prophylaxis in patients with pelvic and acetabular fractures.
Low Dose Unfractionated Heparin (LDUH)
Heparin is a naturally occurring anticoagulant created by mast cells and basophils that inhibits thrombin. LDUH has variable anticoagulant effect, limited bioavailability and highly variable anticoagulant response [26]. LDUH has been used for the prevention and treatment of thrombosis for many years and has been shown to be effective and safe in low to moderate risk general surgical patients. However, ideal strategies for the use of heparin in multiple-trauma patients have not been specifically listed yet [27]. Only one meta-analysis has demonstrated that LDUH was not more effective than no thromboprophylaxis (OR, 0.97; 95% CI, 0.35 to 2.64) [12]. The ACCP reports that LDUH should not be used alone as prophylaxis in trauma patients [28, 29].
Low Molecular Weight Heparins (LMWHs)
LMWHs are derived from unfractionated heparin by depolymerization. LMWHs inactivate numerous coagulation enzymes by binding to antithrombin. As they have lower affinity for binding to proteins other than antithrombin they are, therefore, associated with an expected dose response and less side effects, reducing the incidence of heparin induced thrombocytopenia [7]. Compared with LDUH, LHWHs have reduced protein binding, greater bioavailability, longer half-life and dose-independent clearance, producing a more predictable anticoagulant response without the need of lab monitoring [30]. For those properties LMWHs appear to be the most feasible option nowadays, and have been recommended as the prophylaxis modality of choice for major trauma patients in clinical guidelines [28, 29]. The Eastern Association for the Surgery of Trauma (EAST) guidelines suggests its use is well suited to the polytrauma patient and support a Level II recommendation on the use of LMWHs for patients with pelvic fractures and those patients with an ISS > 9, excluding those with head injuries [21].
LMWHs in present use globally include enoxaparin, dalteparin, nadroparin, tinzaparin, certoparin, reviparin, ardeparin, panaparin and bemiparin. Each LMWH product has a specific molecular weight distribution that determines its anticoagulant activity and duration of effect so one product cannot always be substituted for another. All are recognized as effective methods of anticoagulation. One disadvantage of LMWH is the subcutaneous administration, which could compromise the patient adherence. The use of LMWH, once primary hemostasis has been reached, seems to be the most efficacious and easiest option for the majority of high-risk trauma patients.
A blinded, randomized clinical trial compared enoxaparin with LDUH, both started within 36 hours of injury, including 344 major trauma patients. The LMWH was significantly more efficacious than the LDUH for both DVT (RRR, 30%) and proximal DVT (RRR, 58%) [p = 0.01]. There were no significant differences in the rates of bleeding, need for blood transfusion, or changes in hematocrit [31]. A randomized study of 486 major trauma patients with LMWH or intermittent pneumatic compression devices (IPCD), and weekly DUS screening, was published. Proximal DVT or PE was identified in 3% of the IPCD group and in 1% of the patients with LMWH. Major bleeding was also seen in <2 % of patients in both groups, settling the safety of LMWH in these patients [32].
There is evidence that the early use of LMWH was the only intervention that verified a clear decrease in DVT and PE in pelvic trauma patients. A delay in the administration of thromboprophylaxis by more than 24 hours led to a significant increase in the risk of suffering DVT. Steele et al. concluded that proximal DVT was developed in 3% when LMWH was received within 24 hours of injury, compared to 22% when it was administered more than 24 hours after the injury (p < 0.01) [33]. The authors did not find that the Injury Severity Score (ISS) disturbed the time to administration. They highlight the need to urge prompt administration of LMWH or other effective thromboprophylaxis, however it needs to be investigated with better clinical studies its effect on intraoperative bleeding and perioperative complications.
The immediate use of LMWH in some scenarios as patients with spinal injury and in cases with intracranial haemorrhage remains with out consensus. It sounds logical that if the patient is haemodynamically unstable, it should be delayed its administration until 24 hours after the patient’s condition has stabilized. However, it has been published that the only absolute contraindications to early initiation of LMWH prophylaxis are intracranial bleeding, intraocular haemorrhage, incomplete spinal cord injury concomitant with paraspinal haematoma, constant uncontrolled bleeding and uncorrected coagulopathy. The occurrence of a retroperitoneal hematoma associated with pelvic fracture, is not itself contraindication to LMWH thromboprophylaxis, provided that there is no confirmation of ongoing bleeding [29]. When there is a delay in the first dose of LMWH or the surgery is going to be done more than three days from injury, in order to detect and treat DVT of early onset, it should be considered the performance of preoperative duplex scanning [34]. For these patients with contraindications to LMWH, mechanical modalities should be applied as soon as possible, despite evidence of limited protection.
Some studies suggest that most DVT occur after discharge from hospital [4, 9]. The ideal length of thromboprophylaxis with LMWHs in the multiple-trauma patient is not known, despite its approval. As a general rule it should continue until discharge from the hospital. If we take into account that for patients undergoing major orthopedic surgery (total hip or knee arthroplasties and hip fractures) different guidelines recommend extending thromboprophylaxis in the outpatient period for up to 35 days, as the pelvic trauma patients present even more risk for the development of DVT than the orthopedic group, we should use the LMWHs for at least this period of time, but choices must be made on an individual basis, specially in selected patients with impaired mobility [28, 29].
Oral Vitamin K Antagonist Anti-Coagulants
Warfarin is a coumarin derivative that acts as a vitamin K antagonist. It inhibits the synthesis of the active clotting factors II, VII, IX, and X [35]. Oral anticoagulants have seldom been assessed in the acute phase after major trauma because of their delayed onset of action, multiple drug and food interactions and variable anticoagulant effect among patients that requires regular laboratory monitoring, long duration of effect, difficulty with reversal, potential for bleeding and lower efficacy compared to LMWHs. However, warfarin is used (target INR, 2.5; range, 2.0 to 3.0) in preventing thromboembolic complications beyond the acute phase in some pelvic centers following these criteria: patient with continued thrombosis risk, evidence that hemostasis has been achieved, no further invasive procedures are planned and it is expected that hospitalization and rehabilitation is probable to continue for at least 2 more weeks. Fishmann et al. among 197 patients with acetabular fractures, who received perioperative mechanical prophylaxis followed by 3 weeks for oral anticoagulation, found that the incidence of symptomatic DVT or PE was 4% with no fatal emboli [36].
Aspirin
Aspirin is an irreversible inhibitor of cyclo-oxygenase1, blocking the platelet aggregation for 10 days. It is cheap and no monitoring is required. Its use may have gastrointestinal side effects, allergy and post-operative haematoma growth. Aspirin and other antiplatelet drugs offer much limited protection against VTE compared with other thromboprophylaxis techniques. Those considerations prevented widespread use of aspirin as thromboprophylaxis in the trauma patient [28, 29], so that no studies exist on a large-scale basis in trauma patients to demonstrate its efficacy in pelvic and acetabular fractures.
New Anticoagulant Therapies
Dabigatran, rivaroxaban and apixaban have demonstrated similar levels of efficacy and similar levels of bleeding as enoxaparin in scheduled arthroplasties. Unfortunately these current thromboprophylaxis methods used in orthopedic patients (total hip and knee replacement) have not been specifically studied in pelvic and acetabular fractures, so they should not be use for DVT prevention in these group of patients [28, 37].
Synthetic Pentasaccharides: Fondaparinux
This drug selectively inhibits coagulation Factor Xa and has been indicated to be highly efficacious in the prevention of DVT in patients with hip fractures [38]. Few studies have been done evaluating fondaparinux use in the multiple-trauma patient [39]. Tsiridis et al. reviewed one hundred and eight randomised patients with pelvic or acetabular fractures. The group of enoxaparin had 3% of DVT and 1% of fatal PE. In the group that received fondaparinux there was no documented DVT or PE. The mean number of units of blood transfused was significantly higher in the enoxaparin group (p<0.05). They concluded that post-operative fondaparinux was more effective in decreasing the risk of VTE than LMWH [5]. In another report by these authors the use of fondaparinux, in one hundred and twenty seven patients with pelvic and acetabular fractures, was as effective as enoxaparin and was not related to higher rates of bleeding [40]. However, owing to its long half-life (18 hours) and renal clearance, patients with renal dysfunction can have an accumulation of dose and thus may be at superior risk of bleeding [28, 29]. In contrast to fondaparinux, LMWHs have a shorter half-life (Enoxaparin 4.5 hours) and can be partially reversible with protamine. This reversibility and short half-life of the LMWHs offer a more pragmatic option for patients that frequently required unexpected surgeries and urgent interventional radiology exams.
MECHANICAL PROPHYLAXIS
Compression Devices
Mechanical prophylaxis, as sequential compression devices (SCDs), graduated compression stockings (GCSs) or venous foot pumps (VFPs), is commonly used in lieu of or in addition to pharmacological prevention in patients with pelvic and acetabular fractures, in order to offer additive protection against VTE as well as augmented safety (Fig. 3). However, this dual approach, as it has not been well studied in trauma patients, would increment costs and could get suboptimal compliance with both methods. There is general consensus that efficacy of physical means depends above all on method tolerability, patient collaboration, and adequate application of the procedure. It has been reported an effective compliance of a foot pump device in only 40.2% [41].
This mechanical approach would be especially useful when we have to face a patient with contraindications for chemical prophylaxis (intense intracranial haemorrhage or severe spinal injury, recent or imminent surgery, renal insufficiency, anemia, recent gastrointestinal haemorrhage, active peptic ulcer disease, or liver disease). Under these circumstances, it is suggested that mechanical prophylaxis be used with chemoprophylaxis starting as soon as the contraindication solves. One trial randomized 200 trauma patients to thromboprophylaxis with LMWH started within 48 hours after injury or to VFPs started soon after admission combined with LMWH started 5 days later. There was no significant difference between the two options in bleeding or in DVT rates using magnetic resonance venography before the discharge, supporting the utility of the dual approach, especially useful in trauma patients with an early high bleeding risk [42].
Evidence suggests that more proximal clots are more likely to embolize, being these proximal lower limb DVTs the source of the majority of PEs [43]. Physical methods are mainly intended to reduce venous stasis, but may be insufficient in themselves in moderate or high risk patients, and are generally used combined with pharmacological methods, because although they have been shown to significantly reduce DVT rates, they have a minor effect on preventing proximal DVT. Besides, in the trauma patient, lower limb injuries may exclude the use of mechanical thromboprophylaxis. Fisher et al. evaluated the results of a prospective randomized control trial comparing the use of SCDs with no thromboprophylaxis in seventy-three patients with pelvic and acetabular fractures, without detecting difference in the incidence of DVT or PE. Only a trend to lower DVT rates was seen in the SCDs group, but no effect on PE was seen [44]. Rogers et al. noted that their efficacy in trauma patients has not been appropriately demonstrated and may be correlated with complications, thus suggested a Level III recommendation on the use of pneumatic compression devices for thromboprophylaxis [21].
INFERIOR VENA CAVA FILTERS
In high-risk patients when contraindications to anticoagulation are present or when anticoagulation fails, prophylactic inferior vena cava filters (ICVFs) can be used to prevent pulmonary emboli as a common last resort (Fig. 4). These endovascular devices do not prevent the development of a deep vein thrombus; they interrupt the flow in the inferior vena cava to prevent the most significant sequel of DVT, the life-threatening pulmonary embolus. Filters have been related as preventing PE in the existence of proven lower limb DVT in 98% of cases [17]. The best position is in the infra-renal IVC, covering prophylaxis against infra-renal thrombosis as well as PE. IVC filter has a number of published complications associated with its insertion, such as vessel injury, and longer-term problems including filter migration, IVC thrombosis and recurrent DVT [45-48]. Filters may be either permanent or retrievable. The latter are becoming an increasingly attractive option as non-permanent filters offer immediate PE prophylaxis, but can be removed, reducing the long-term complications associated with permanent devises. Filters become progressively challenging to remove, the longer they are inserted [49]. As the vast majority of the trials are retrospective, without a comparative group, the evidence available for the use of IVC filters in trauma patients is self-contradictory [49, 50]. In a cohort of 51 patients with acetabular fractures, while the no-filter group had a fatal PE, the discrepancy in clinical pulmonary emboli was not statistically significant. During their insertion no complications were informed in this review [51]. A multicenter, randomized trial assessed the additional benefit of IVCF to anticoagulation in patients with proximal DVT. Although there were fewer PE in the filter group, mortality was not reduced and the patients with filter and anticoagulation showed a remarkable tendency to recurrent DVT [52].
(A)
Although there is diverse data regarding the safety and retrievability of IVCFs, the Eastern Association for the Surgery of Trauma has published the indications for insertion of IVC filter in trauma patients (Table 1) [21]. In contrast, the ACCP guidelines do not recommend the use of IVC filters as thromboprophylaxis for high-risk patients unless there exists both a proven proximal DVT and also absolute contraindication to full dose anticoagulation or urgent surgery [28, 29]. In the common scenario of patient who requires definitive pelvic surgery and has evidence of a proximal DVT on the preoperative venous ultrasound examination, it is recommended to insert a temporary filter before surgery, starting anticoagulants postoperatively as soon as it is safe to do so, removing the filter within the first month, when the patient has been fully anticoagulated [53, 54].
GUIDELINES
Several practice management guidelines have been published to prevent and detect venous thrombosis. The key point of using guidelines is to offer a better care to our patients by decreasing the risk of postoperative VTE without increasing the chance for other complications, such as bleeding. The American College of Chest Physicians (ACCP) provides the most comprehensive thromboprophylaxis guidelines so as to help the clinicians with their prophylactic decisions. The 8th Edition of the ACCP Evidence-Based Clinical Practice Guidelines, reviews the prevention of venous thromboembolism (VTE) in different scenarios, and they give some suggestions for major trauma patients [29]. Their different recommendations were settled after studying the evidence for prophylaxis in all these patients. Grade 1 recommendations indicate that the benefits do or do not compensate risks and costs, however grade 2 suggestions denote that individual case values may lead to different alternatives. For all major trauma patients, this guideline recommends routine thromboprophylaxis if possible (Grade 1A). In the absence of a major contraindication, they recommend using LMWH thromboprophylaxis starting as soon as it is considered safe (Grade 1A). An acceptable alternative would be the dual administration of LMWH and the optimal use of a mechanical method of thromboprophylaxis (Grade 1B). If LMWH thromboprophylaxis is contraindicated due to active bleeding or high risk for bleeding, they recommend that mechanical thromboprophylaxis with IPC or possibly with GCS alone be used (Grade 1B). When the bleeding risk decreases, they recommend that pharmacologic thromboprophylaxis be substituted for or added to the mechanical thromboprophylaxis (Grade 1C). This review also recommend against routine Doppler ultrasonography (DUS) screening for asymptomatic DVT (Grade 1B). They do recommend DUS screening in patients who are at high risk for VTE and who have received suboptimal or no thromboprophylaxis (Grade 1C). Regarding the IVCF as thromboprophylaxis they recommend against its use (Grade 1C). Concerning the length of prophylaxis they recommend the continuation of thromboprophylaxis until hospital discharge (Grade 1C). If the patients present impaired mobility and undergo inpatient rehabilitation, they suggest continuing thromboprophylaxis with LMWH or a VKA (Grade 2C).
The chapter on the prevention of VTE in patients with major trauma in the 9th edition contains numerous changed recommendations, in comparison with the 8th publication of the ACCP. The authors found reasons for the downgrading of most recommendations to grade 2C [28]. For major trauma patients, they suggest the use of LDUH (Grade 2C), LMWH (Grade 2C), or mechanical prophylaxis, preferably with IPC (Grade 2C), over no prophylaxis. For patients at high risk for VTE (including those with acute spinal cord injury, traumatic brain injury, and spinal surgery for trauma), they suggest adding mechanical prophylaxis to pharmaco-logic prophylaxis (Grade 2C) when not contraindicated by lower-extremity injury. If LMWH and LDUH are contraindicated, they suggest mechanical prophylaxis, preferably with IPC, over no prophylaxis (Grade 2C) when not contraindicated by lower-extremity injury. They suggest adding pharmacologic prophylaxis with either LMWH or LDUH when the risk of bleeding diminishes or the contraindication to heparin resolves (Grade 2C). They suggest that an IVC filter should not be used for primary VTE prevention (Grade 2C). Last but not least, they suggest that periodic surveillance with venous compression ultrasound should not be performed (Grade 2C). Geoghegan el al. formulated a questionnaire requesting information on thromboprophylaxis and surveillance protocols used in the pelvic units of United Kingdom. Mechanical thromboprophylaxis was used in 67% of the units. No unit routinely used prophylactic IVC filters. Chemical thromboprophylaxis was routinely used in 100% of the centers. 95% used prophylactic doses of LDH or LMWH. Clinical surveillance alone for thromboembolism was employed in 90% of the centers and only 10% of the units routinely performed radiological surveillance with ultrasound Doppler, pre-operatively. These data are in line with the recommendations given by the ACCP [55].
The ACCP are evidence-based clinical practice guidelines that were established in a methodical fashion. The heterogeneity of including all trauma injuries, lack of data from well-designed studies and few studies available in the literature concerning the prevention of VTE in patients with pelvic and acetabular fracture, most of them from observational studies, prevents surgeons from warranting these recommendations are relevant to patients with pelvic fractures and emphasizes the necessity for further investigation in this high-risk population [56]. This problem was documented by the National Institute of Clinical Excellence (NICE) in its 2012 guidelines update on decreasing the risk of VTE, concluding that the most practical approach of thromboprophylaxis in patients with pelvic and acetabular fractures remains uncertain from the existing evidence [57].
In the absence of prospective randomised controlled trials, VTE prevention policies in different institutions will not be homogeneous.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


