All published articles of this journal are available on ScienceDirect.
Resuscitation of Polytrauma Patients: The Management of Massive Skeletal Bleeding
Abstract
The term ‘severely injured patient’ is often synonymous of polytrauma patient, multiply-injured patient or, in some settings, polyfractured patient. Together with brain trauma, copious bleeding is the most severe complication of polytrauma. Consequently hypotension develop. Then, the perfusion of organs may be compromised, with the risk of organ failure. Treatment of chest bleeding after trauma is essential and is mainly addressed via surgical manoeuvres. As in the case of lesions to the pelvis, abdomen or extremities, this approach demonstrates the application of damage control (DC). The introduction of sonography has dramatically changed the diagnosis and prognosis of abdominal bleeding. In stable patients, a contrast CT-scan should be performed before any x-ray projection, because, in an emergency situation, spinal or pelvic fractures be missed by conventional radiological studies. Fractures or dislocation of the pelvis causing enlargement of the pelvic cavity, provoked by an anteroposterior trauma, and in particular cases presenting vertical instability, are the most severe types and require fast stabilisation by closing the pelvic ring diameter to normal dimensions and by stabilising the vertical shear. Controversy still exists about whether angiography or packing should be used as the first choice to address active bleeding after pelvic ring closure. Pelvic angiography plays a significant complementary role to pelvic packing for final haemorrhage control. Apart from pelvic trauma, fracture of the femur is the only fracture provoking acute life-threatening bleeding. If possible, femur fractures should be immobilised immediately, either by external fixation or by a sheet wrap around both extremities.
1. INTRODUCTION
The accurate definition of severely injured patients is a question of crucial importance, and studies must be conducted to identify a common pattern so that useful conclusions be drawn regarding diagnosis and management. The term ‘severely injured patient’ is often synonymous of polytrauma patient, multiply-injured patient or, in some settings, polyfractured patient. To achieve a precise classification in this respect, scores have been developed, with the Injury Severity Score (ISS) [1], the Abbreviated Injury Scale (AIS) [2], the Revised Injury Severity Score (RISS) [3] and the New Injury Severity Score (NISS) being the most commonly used. New internet versions of these, enabling their rapid calculation, have also been developed [4]. Nonetheless, after studying a database of more than 40,000 patients, the German Trauma Society concluded that greater precision is required, and that an anatomically-based definition is needed in order to identify patients who are critically ill [5]. Many such definitions have been proposed, but the cornerstone of polytrauma definition is generally considered to be the existence of an AIS score of over two in at least two ISS body regions (2xAIS score>2) [6]. Theimportance of this issue is highlighted by the fact that severely injured patients have higher mortality rates, present more frequent intensive care unit admissions and require longer hospital stays.
Together with brain trauma, copious bleeding is the most severe complication of polytrauma. Massive bleeding is an acutely life-threatening complication in major trauma requiring rapid and accurate diagnosis and treatment. Exsanguination by massive bleeding is the product of vascular injury, either intra or extra-visceral, or of bone fractures. The most common situation leading to massive bleeding is trauma, with about forty per cent of trauma-related mortality being due to uncontrolled haemorrhage [7]. The basis of the treatment of major bleeding is surgical management together with the infusion of fluids and blood. The European Medicines Agency recommends that hydroxyethyl starch solutions (HES) should not be used for the treatment of hypovolemic shock in sepsis patients and that their use in haemorrhagic shock patients should be limited to cases when crystalloids alone are not sufficient [8].
Since infusion may provoke further complications, particularly when coagulation abnormalities are present, surgical manoeuvres for bleeding control become critical.
1.1. Surgical Resuscitation
The purpose of resuscitation is to prevent a fatal outcome. When bleeding occurs, less fluid circulates within the bloodstream, and consequently hypotension develops. Then, the perfusion of organs can be compromised, with the risk of organ failure. Tissue oxygenation, particularly of main organs such as the brain, kidneys and liver, is essential to maintain good health status. In this respect, a systolic blood pressure of 80-90 mm Hg guarantees adequate perfusion. During the early phases of massive bleeding, it is fluid that is missing, and there is strong evidence that treatment of the hypotensive bleeding trauma patient should start with crystalloids, and also that hypotonic solutions such as Ringer’s lactate should not be given to patients with severe brain trauma [9]. Fluid resuscitation restores intravascular volume, as the first-line therapy, and also corrects hypotension. However, this strategy of low-volume resuscitation needs to be balanced considering the severity of haemorrhage and the time elapsed during transfer of the patient, in order not to provoke a hypovolemic shock. To avoid a “lethal triad”, fluid resuscitation should be guided as soon as possible by haemodynamic monitoring to optimise its adequacy with respect to tissue perfusion [10].
The term “lethal triad” refers to the end point of a severe course of the combination of coagulopathy, hypothermia and acidosis, in the evolution of exsanguinating patients [11-13]. In this respect, however, a recent Cochrane review of randomised controlled trials found no evidence for or against using an early or larger volume of intravenous fluid administration to treat uncontrolled haemorrhage [14]. It is currently believed that the use of norepinephrine improves blood pressure values, thus reducing the need for fluid therapy and preventing overload, haemodilution and coagulopathy [15-17].
Although fluid infusion is still considered the cornerstone of acute treatment of hypovolemic shock, this should be reconsidered if the haemorrhage continues. In any case, this approach remains controversial, and stopping the bleeding should be the physician’s main priority. In cases of acute trauma, persistent systolic blood pressure of less than 90 mm Hg after treatment with vasoactive drugs is acknowledged to be a sign of active bleeding, requiring a surgical manoeuvre, until proven otherwise. Of course, the time elapsed between the injury event and surgical operation to control bleeding should be as short as possible. In the case of open haemorrhage of a limb, the use of a tourniquet to stop life-threatening bleeding is strongly recommended, and tourniquet-induced pain should be disregarded. However, whenever possible, the tourniquet should be released within two hours in order to avoid nerve paralysis and limb ischaemia [9], and be followed by the application of local compression.
Since more complex surgical manoeuvres than simple limb tourniqueting are needed, and they must be hospital-delivered, transportation from the accident site to the hospital is a key issue. Studies of helicopter transportation for major trauma patients have been conducted, but evidence-based results have yet to confirm exactly which elements make it advisable for patients to be transported by helicopter [18]. Different studies have reported conflicting results concerning the cost and effectiveness of helicopter emergency medical services; moreover, the fact that diverse methodologies have been used makes it very difficult to conduct a systematic review of this question [19]. On the other hand, the cost of overtriage must be taken into account. It has been shown that one third of low-risk injured patients are taken to major trauma centres, thus provoking unnecessary expense [20]. Therefore, while it is undoubtedly very important that patients should be rapidly transported to hospital for surgical treatment to be performed in case of major bleeding, the optimum approach to this question remains unknown.
After proper medicalised transportation, once the patient is in hospital, it should be taken into account that there are five potential sources of major blood loss: large skin lesions, chest injuries, abdomen injuries, and bone fractures of the pelvis or the lower extremities (Algorithm 1).
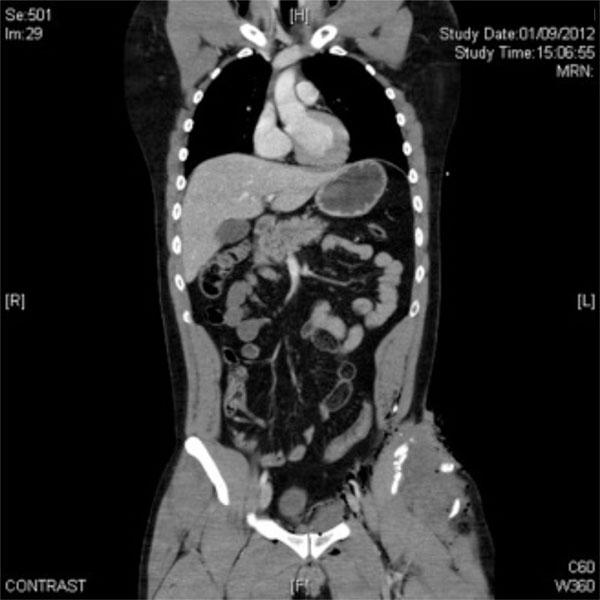
A 29-year-old male patient admitted to the emergency unit of our hospital after a motorcycle accident. Contrast CT-scan showed no chest or abdominal bleeding but there was a stable left iliac crest open fracture with regional swelling.
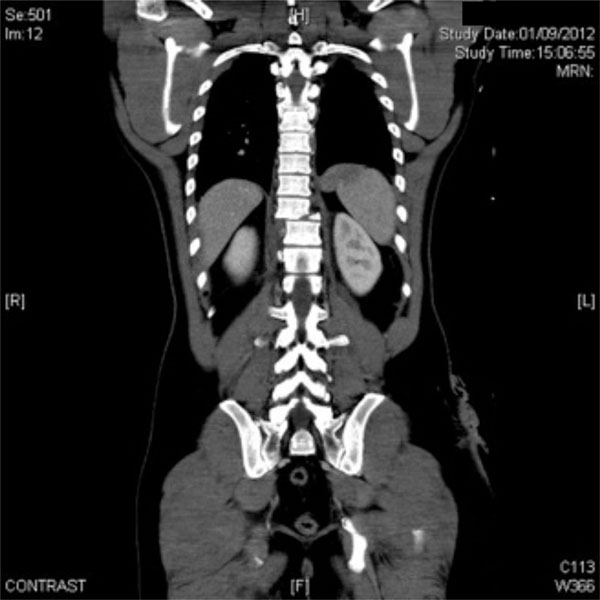
The same patient. CT-scan, coronal projection: T9 instable fracture with no neurological syndrome.
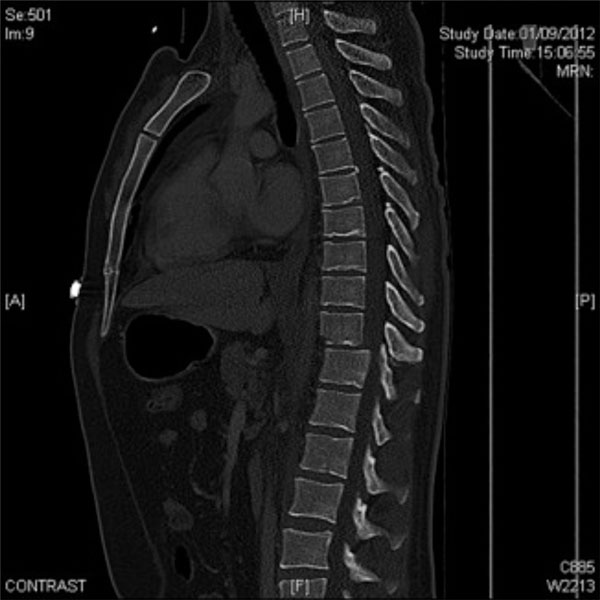
The same patient. CT-scan, sagittal projection: T9 fracture with traumatic spondylolisthesis. This patient also presented an open right tibial fracture.
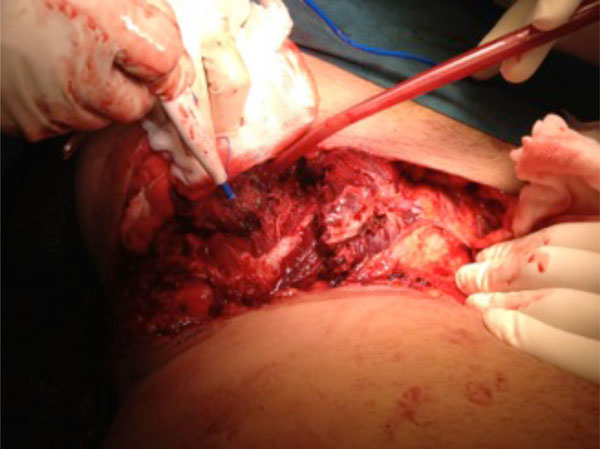
The same patient. Debridement of the iliac crest fracture, and haemostasis.
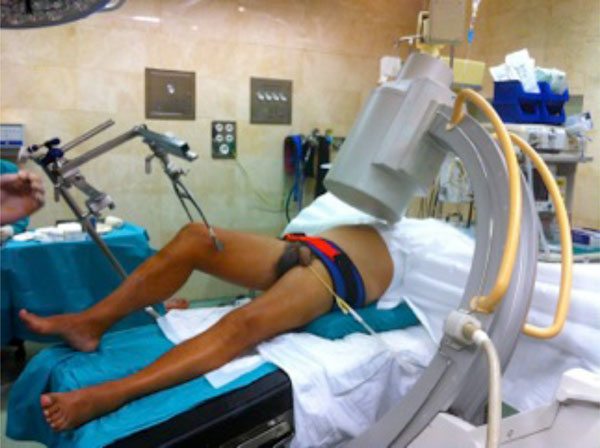
A 32-year-old male patient with an open book rotationally unstable pelvis fracture with a pelvic binder until just before undergoing iliosacral screwing, and anterior pubis plate fixation.
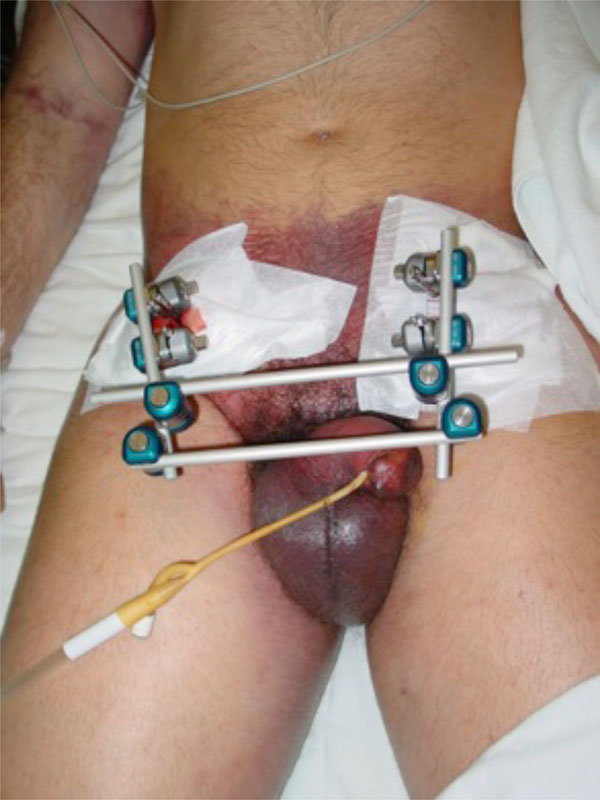
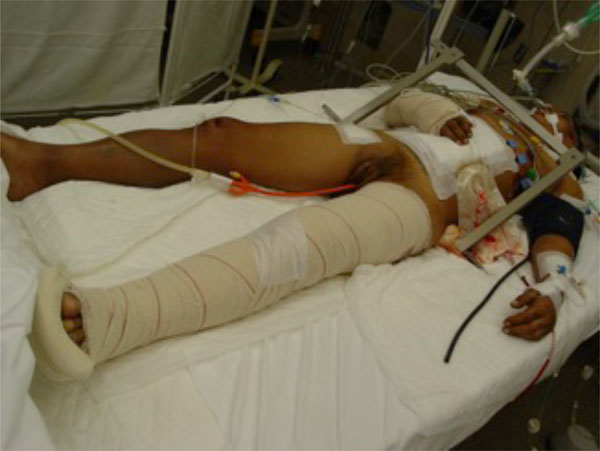
A 45-year-old male patient with a bilateral open book pelvis fracture stabilised with external fixation. Subcutaneous haemorrhage shows the severity of pelvic bleeding. The fixator pins are inserted just above the anteroinferior iliac spine. The rods leave enough room for a subsequent anterior abdominal approach.
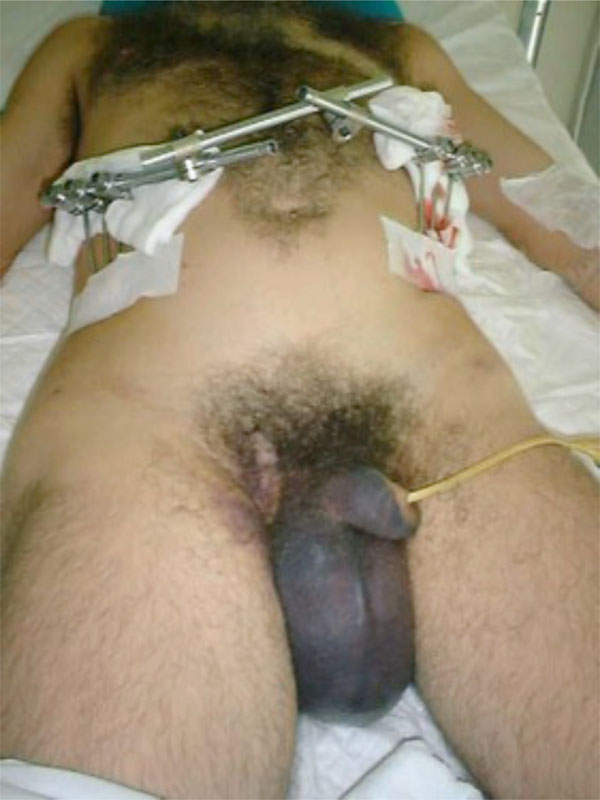
A 38-year-old male patient with a left vertical unstable pelvic fracture. Stabilisation with external fixation, seeking to leave enough room for laparotomy. However, iliac crest pin purchasing provides a less robust fixation.
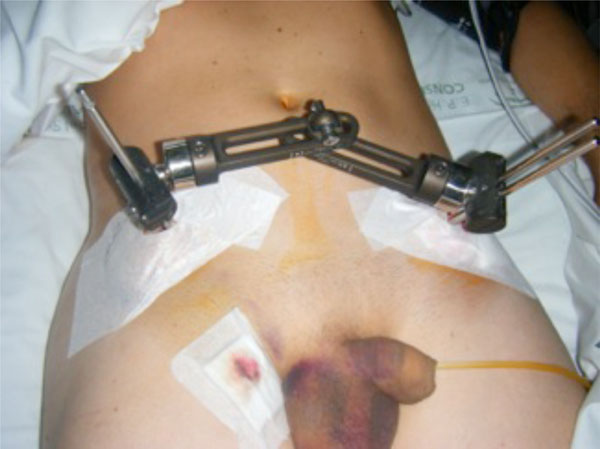
A 28-year-old male patient with an open book rotationally unstable pelvic fracture stabilised with external fixation purchased just above the anteroinferior iliac spine. However, the connection between the two sides interferes with a possible future laparotomy.
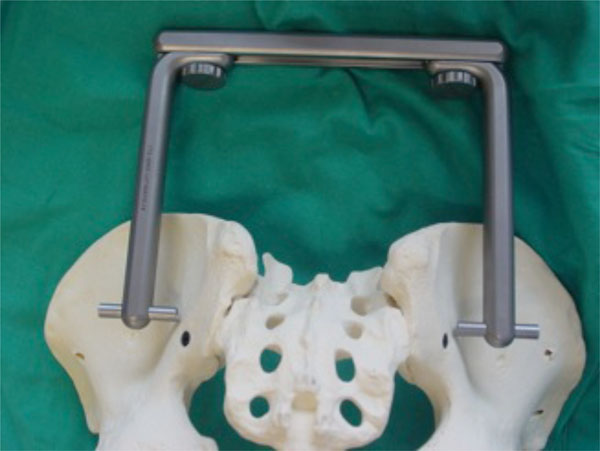
Phantom of a C-clamp fixation of sacroiliac joints.
A 28-year-old woman admitted to the emergency unit after a motorcycle accident, presenting a right C-type vertically unstable pelvic fracture together with a knee bone fracture. C-clamp stabilisation, in addition to a laparotomy and a colostomy. Left lower limb was stabilised with plaster.
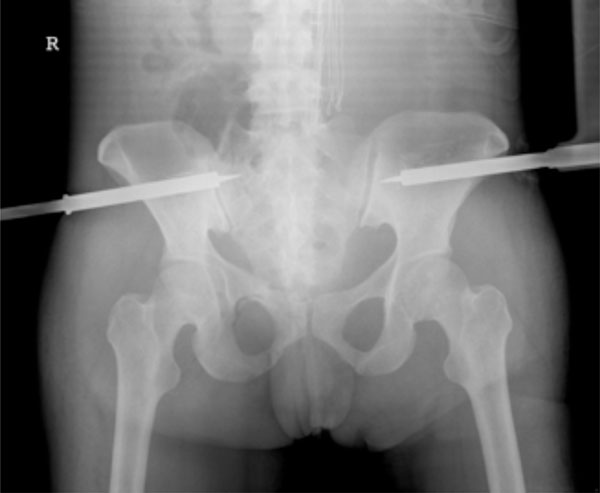
The same patient. X-ray control of the pelvic stabilisation.
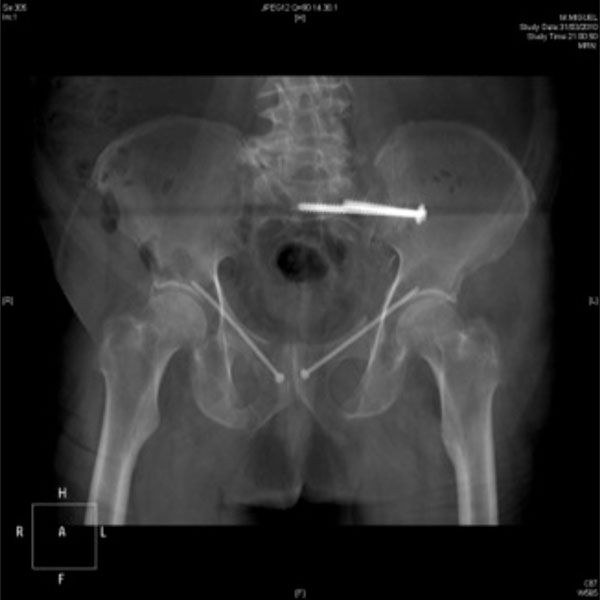
Percutaneous left iliosacral and bilateral anterior column screws to stabilise a bilateral C-type unstable fracture.
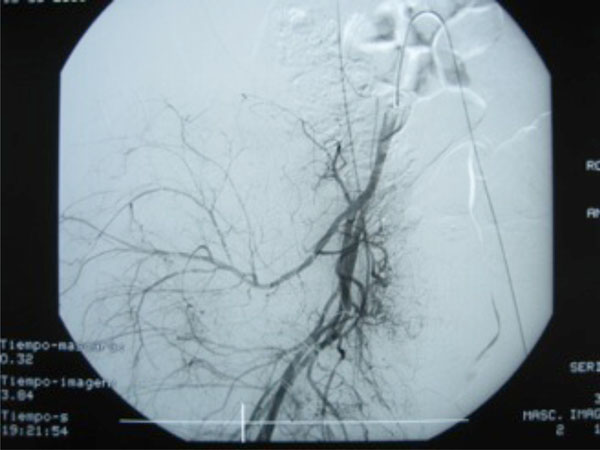
Retrograde left-to-right femoral to aorta-iliac angiography.
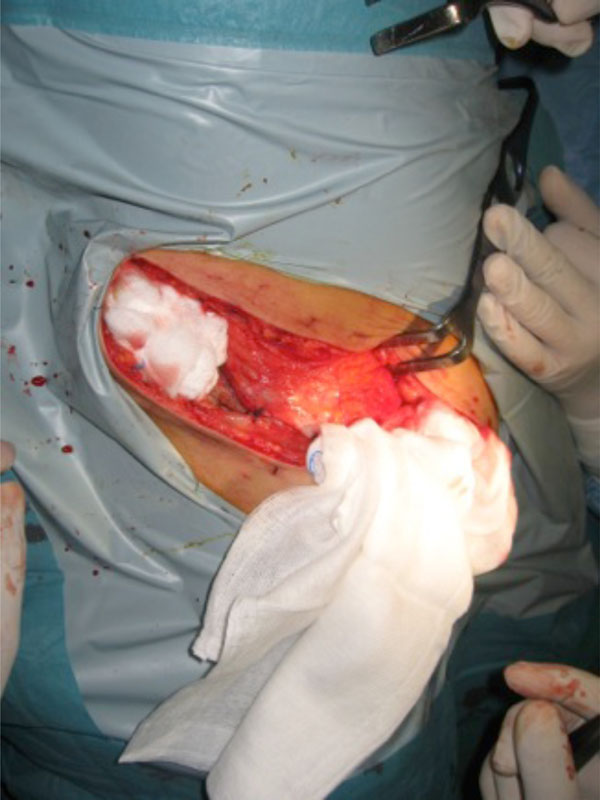
Packing in an open pelvic fracture after enlarging the wound for debridement.
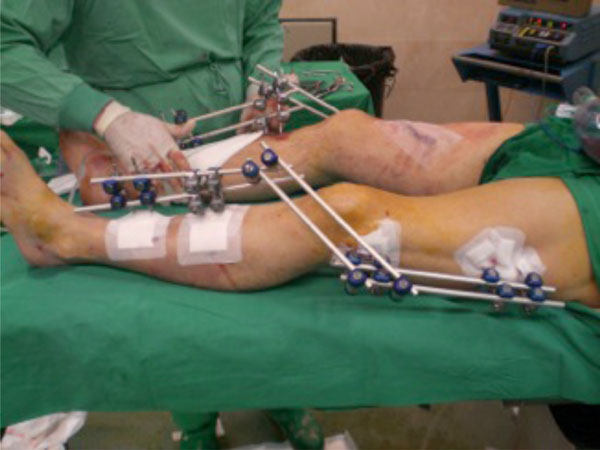
Fixation of bilateral femur and tibial fracture with a span fixator femur to tibia.
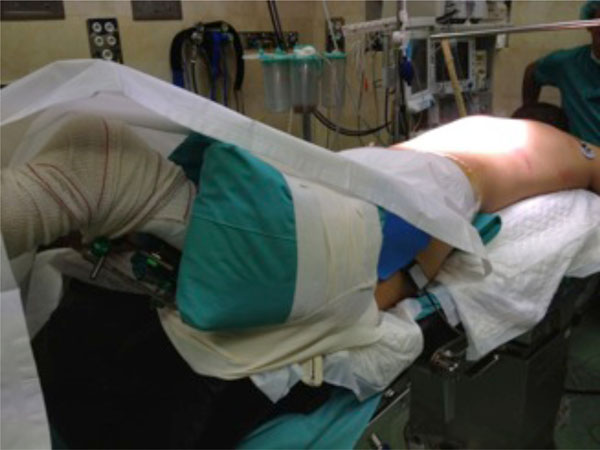
Spinal fracture surgical stabilisation of the patient shown in Figs. (1-4), applying the damage control concept to the spine. The right open fracture has been debrided and stabilised with an external fixation.
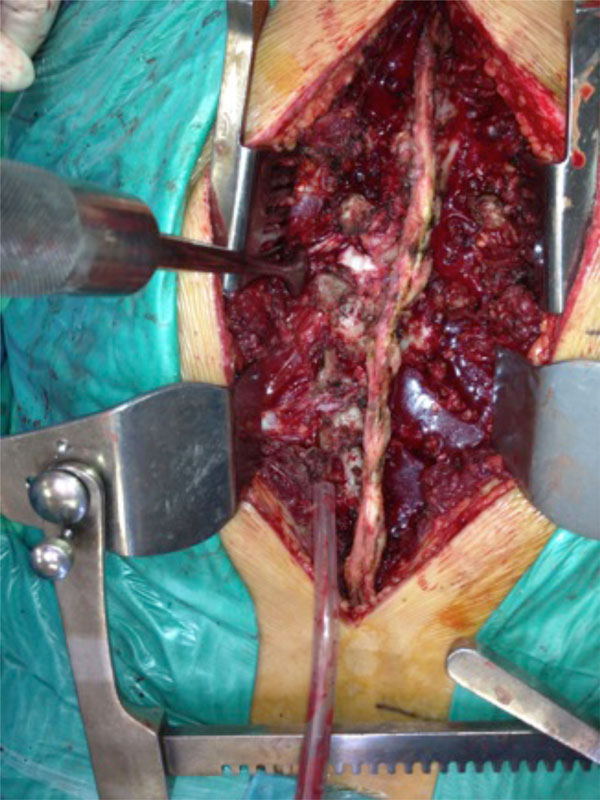
The same patient. Surgical field of the spine. Articular dislocation with lateralisation can be seen.
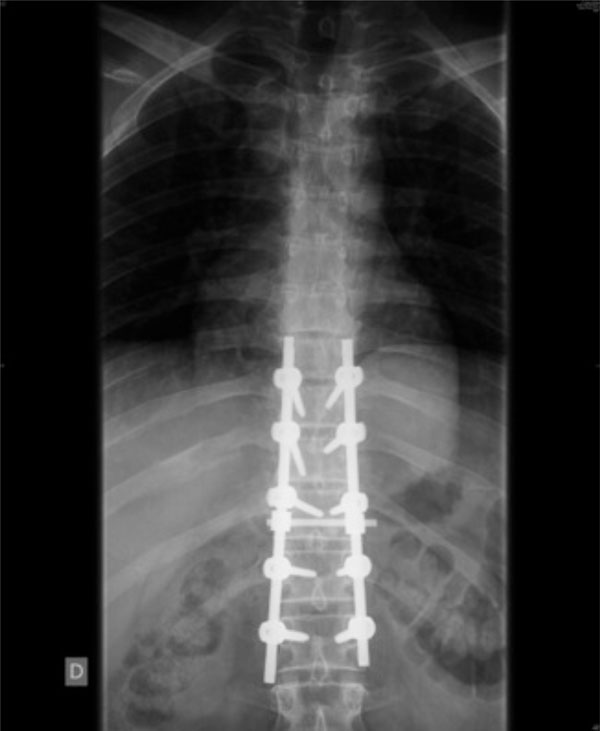
The same patient. Anterior-posterior X-ray control.

The same patient. Lateral X-ray control.
1.2. Chest and Abdominal Bleeding
Severe thoracic trauma is present over 80% of polytrauma patients and is associated with a mortality rate of nearly 40% [21]. Chest wall injuries are frequently accompanied by major vessel lesions and the resulting massive haemorrhage may be fatal. Nonetheless, the severity of chest injuries varies according to which structures are damaged; whereas lesions of the intrapericardial aorta and left cardiac ventricle are especially severe, atrial bleeding of the right ventricle can course in a benign manner. Diaphragm, lung and mediastinum injuries can provoke very severe chest traumas.
Contrast CT-scan is the most important diagnostic tool to achieve a prompt diagnosis of chest bleeding. A multicentre study of about 4,500 severe trauma patients obtained a standardised morbidity ratio, based on the trauma injury severity score, of 0.745 for patients given a whole-body CT scan versus 1.034 for those given non-whole-body CT. Multivariate adjustment for hospital level, year of trauma and potential centre effects confirmed that whole-body CT is an independent predictor for survival [22].
Ultrasound is another important diagnostic facility, providing widespread and rapid availability, reproducibility and reliability for the detection of haemothorax [23], although it is not as reliable as contrast CT-scan. Therefore, suspicion of major chest bleeding is an indication for immediate contrast CT-scan [22]. After full diagnosis, the classification of thoracic trauma is very important in relation to survival prognosis. Many such classifications have been described, most of which are CT-scan based [24]. Nevertheless, the sensitivity, specificity and complication prediction of this instrument have yet to be comprehensively studied.
Treatment of chest bleeding after trauma is essential and is mainly addressed via surgical manoeuvres. These must aim to achieve rapid control of the primary haemorrhage, leaving reconstructive surgery for a secondary operation when the patient is in a better clinical situation. As in the case of lesions to the pelvis, abdomen or extremities, this approach demonstrates the application of damage control (DC).
Abdominal injuries are also a very common potential major source of bleeding. Focused abdominal sonography for trauma (its acronym, FAST, stresses the necessity of rapid performance), peritoneal aspiration with lavage, contrast CT-scan and laparoscopy are the main diagnosis facilities used when abdominal bleeding is suspected.
Although peritoneal aspiration and lavage are still recommended by some authors, the introduction of sonography has dramatically changed the diagnosis and prognosis of abdominal bleeding. In a multinational study of nearly 5,000 patients with multiple trauma - 65% with cerebral injuries, 58% with thoracic trauma and 81% with extremity fractures - the authors compared three sequenced decade periods, during which pre-hospital care became more aggressive, with an increased use of intravenous fluid resuscitation and reduced rescue time. For the initial clinical diagnosis of massive abdominal haemorrhage, ultrasound (decade I: 17%, decade II: 92%, and decade III: 97%), replaced peritoneal lavage (decade I: 44%, decade II: 28%, decade III: 0%), and CT-scans were used more frequently for the initial diagnosis of head injuries and other injuries of the trunk throughout the observation time. Acute renal failure decreased by half (decade I: 8.4%; decade II: 3.7%, decade III: 3.9%), acute respiratory distress syndrome initially decreased but increased again in the last decade (I: 18.1%, II: 13.4%, III: 15.3%), and the rate of multiple organ dysfunction syndrome increased continuously (I: 14.2%, II: 18.9%, III: 19.8%) probably due to a decline of the mortality rate from 37% in the first decade, to 22% in the second, and 18% in the third, with a parallel increase in the time of death [25]. Therefore, positive FAST in the absence of patient haemodynamic instability should be followed by contrast CT-scan to maximise diagnostic accuracy [22].
CT-scan is an excellent tool for the diagnosis of solid abdominal organs (liver, spleen and kidney) and of blood-vessel lacerations, which in the case of abdominal bleeding are the main source of haemorrhage. However, it has limited sensitivity for the diagnosis of intestinal injuries. Lesions to the major abdominal vessels require a speedy surgical approach for reconstructive surgery, whereas smaller arteries can be treated by embolisation. Subcapsular haemorrhage of solid organs can be treated conservatively with bed rest.
With respect to abdominal vascular bleeding, DC surgery seeks to avoid the “lethal triad” of hypothermia, acidosis and coagulopathy. In a study comparing the current situation with that of 30 years ago it was found that although exsanguination still provokes a high rate of mortality in these patients, DC surgery together with the implementation of a massive transfusion protocol has decreased the mortality dramatically. This study found that initial ph decreased from 7.21 to 6.96 in patients with overt coagulopathy due to the recovery of more severe patients who would have died years ago, and that the administration of clotting factors did not change overall mortality [26].
Apart from surgical manoeuvres on the chest and abdomen, further DC surgical methods should be carried out in patients with deep haemorrhagic shock, signs of ongoing bleeding and coagulopathy (evidence grade 1B) [9]. Acidosis and hypothermia are also situations that require prompt abdominal surgery as a DC procedure (evidence grade 1C) [9]. Ideally, patients should be operated on while they are still haemodynamically stable (evidence grade 1C) [9] and before the lethal triad develops. The use of topical haemostatic agents by packing, in combination with other surgical actions to the liver, spleen or any other organ where bleeding taking place, is also very useful (evidence grade 1B) [9].
2. PELVIS
The skeleton can also be a major source of bleeding. In particular, pelvis and femur fractures provoke a very dangerous and even fatal haemorrhage. High-energy pelvis fractures usually produce haemodynamic instability related to direct blood loss from broken bones, ruptured major vessels or vascular plexuses involved in pelvic trauma. Understanding the anatomy and pathomechanics of these situations is essential for prompt diagnosis and appropriate treatment.
Pelvic fracture can be the most important source of major bleeding after trauma; the initial diagnosis is based on clinical suspicion. Limb shortening, together with abnormal movements of the pelvic bones on examination and, in many cases, visible subcutaneous haematoma are all grounds for this clinical diagnosis. The definitive diagnosis is obtained by contrast CT-scan including head-to-pelvis slices [22]. In severely traumatised haemodynamically stable patients, a contrast CT-scan should be performed before any x-ray projection, because, in an emergency situation, spinal or pelvic fractures can be missed by conventional radiological studies [27]. In summary, contrast CT-scan, apart from bone lesions, identifies any source of bleeding, and is coming to be the first-choice diagnostic tool for severe trauma patients (Figs. 1-4).
Fractures or dislocation of the pelvis causing enlargement of the pelvic cavity, provoked by an anteroposterior trauma, and in particular cases presenting vertical instability, are the most severe types and require fast stabilisation by closing the pelvic ring diameter to normal dimensions and by stabilising the vertical shear [28].
2.1. Ring Closure
2.1.1. Binders
Pelvic binders are very useful for ring closure, dramatically reducing mortality rates. The binder should be positioned at the height of the greater trochanter for better haemorrhage control, better access to the abdomen and greater patient comfort (Fig. 5) [29]. Its application is quick and easy and therefore this should be done in the emergency room before the patient is transferred for CT-scan [30]. As an alternative to binders, a conventional sheet can be applied [31], and this method is apparently more cost effective [32]. In a study conducted using embalmed whole human cadavers, electromagnetic sensors were applied to each hemipelvis in order to record sagittal, coronal and axial rotation during application of the device, simulating bed transfer, log-rolling and head of bed elevation. The authors observed no significant differences in displacements when the pelvis was immobilised with either a sheet or a pelvic binder [32]. In another study, the same authors found no differences, either, when external fixation was used [33]. Although some studies have reported that binders present a statistically better control of symphisis pubis diastasis than sheets, in Burgess and Young anterior-posterior compression type II pelvic injuries (Tile types B-1 and C) [34] both binders and sheets obtain comparable results [30, 31], without overreduction [30]. In a systematic review, pelvic circumferential compression devices, such as binders, were found to be very effective, although they can provoke skin sores [35]. Furthermore, pressure exerted on the skin by
binders can exceed the tissue-damage threshold, an outcome that is influenced by the patient’s body mass index, waist size and age. To date, no studies have been published as to how long a binder can be left in place without provoking sores. Nevertheless, it seems advisable not to remove it while the patient is still at risk of haemorrhage [30], which is usually for about two days.
Despite the effectiveness of binders, their use is not as widespread as might be expected. In a national survey of 144 trauma units in the United Kingdom, which obtained a 100% response rate, it was found that only half of the orthopaedic departments and registrars had participated in a training programme on pelvic binder application, and that emergency departments reported an even lower proportion. The greater trochanter was identified as the most suitable site for the binder. In nearly all cases in which the pelvic binder was properly managed, it was not removed after imaging studies revealed the patient to be haemodynamically stable. These authors concluded that specific programmes should be implemented to foster and inform about the use of pelvic binders [36]. In any case, binders have definitively replaced the military antishock trousers (MAST) commonly used during the 1970s and 80s, which could cause compartmental syndrome of the lower limbs.
2.1.2. External Fixation
External fixation provides the same effect as binders but with certain additional advantages; it allows laparotomy, thus obtaining greater mechanical stability, and avoids anterior compression to the abdominal cavity and also the risk of skin necrosis that arises when the binder is kept in place for several days (Figs. 6-8) [30]. On the other hand, binders are indicated at the time of admission of very acute cases, as external fixation is a more time consuming and aggressive technique, requiring anaesthesia and an operative room. Moreover, external fixation can be an upsetting technique for patients. Attempts have been made to avoid the infection of pin tracks and the discomfort caused by maintaining the device in a very uncomfortable area by the use of subcutaneous fixation. The long-term benefit or otherwise of this technique has yet to be established [37]. In a multicentre retrospective study of 91 patients, the authors observed a lesion of the lateral femoral cutaneous nerve in 27 patients, and heterotopic ossification around implants in 32 of 91 patients [38]. Furthermore, a comparative study of the use of subcutaneous versus conventional anterior external fixation showed that subcutaneous fixation presented fewer wound complications and less morbidity than conventional constructs. No reduction was lost with either technique [39]. In any case, the conversion of external to internal fixation should be performed as soon as possible, both in civil and in military medical treatment [40].
Anterior external fixation frames provide poor stabilisation for posterior pelvis ring lesions, and therefore posterior fixation is advisable [41]; this can be provided either by a C-clamp device [42, 43] or by iliosacral screwing [44-47]. These and other techniques have been widely applied, and the results published by many authors (Figs. 9-12). The application of a C-clamp in cases of anterior fracture patterns, particularly type-APC-2 fractures according to the Young and Burgess classification [34], has also been recommended to enhance systolic blood pressure and to obtain good bone fracture reduction [48]. However, and surprisingly, it is still not known which method is most effective for certain fracture patterns, such as transverse sacral fractures, as the wide range of patient types and results makes it very difficult to draw solid conclusions, even from systematic reviews [49]. C-clamps and iliosacral screwing can only be applied when the iliac bone is preserved - in pure iliosacral dislocation or in sacral fractures - and these techniques are more difficult to perform during an emergency situation [44-47]. The combination of CT-scan-based temporary reduction fixation followed by iliosacral screwing has also been used [50]. It should be noted that, even when applied by skilled practitioners, a C-clamp provoke iatrogenic lesions, such as migration into the pelvic cavity and subsequent intestinal piercing, or further bone fracture [42, 43].
Because of pelvic dysmorphism and the tension that arises during critical situations, iliosacral screwing in an emergency is a technically demanding technique even for experienced surgeons [43, 45, 51]. Both C-clamping and iliosacral screwing must be conducted with the help of fluoroscopy or CT-scan (however, the latter resource is not always available) [46]. In any case, during the application of external fixation, whether by means of an anterior frame or a C-clamp device, care must be taken to allow enough room to manoeuvre for an eventual laparotomy (Figs. 6-8).
Since prompt C-clamp application in a patient who is bleeding be troublesome and can also provoke severe iatrogenic complications [51], many attempts have been made to construct an anterior frame for posterior compression, seeking to close the sacroiliac complex in an efficient manner [41]. However, reproducing research findings in practical situations is still a problematic issue [52].
2.2. Embolisation or Packing?
2.2.1. Embolisation
Although pelvic ring closure, using binders or fixators, provides a tamponade effect [28], together with a fracture occlusion which facilitates the cessation of haemorrhage, this technique can fail, because the extraperitoneal cavity pressure achieved by ring closure may be less than the bleeding pressure. In any case, before any open management of abdominal bleeding is attempted, pelvic stabilisation (closing the pelvic ring) must be achieved. This is essential not only for haemodynamic stability but also to facilitate surgical manoeuvres within the abdominal cavity. Should pelvic ring closure fail to produce haemostasis, further rapid action is needed. If haemodynamic instability persists after pelvic ring closure, either angiography or retroperitoneal packing must be performed. To do so, conducting a prior contrast CT-scan will enable accurate diagnosis [53, 54]. In highly critical situations of severe haemodynamic instability, the patient should be taken straight to the operating theatre.
Angiography with embolisation is more commonly needed in anteroposterior open book-type or vertical fracture patterns, although the morphology of the fracture does not provide a complete predictive value of the vascular lesion and the consequent bleeding [55]. Angiography is a less invasive technique, and can be carried out straightforwardly by an experienced interventional radiologist (Fig. 13). Although it is better performed when the pelvis has been stabilised, it can be carried out even in an unfixed pelvis [56]. Angiography, however, is only appropriate to deal with arterial bleeding - and even in this case, not the bleeding provoked by major vessels - and is a very time consuming technique [53], particularly in anteroposterior bilateral lesions [57]. Since the main source of bleeding in pelvic fractures is the veins, and from the bone fracture site, the validity of angiography in high energy pelvic fractures is very limited. Furthermore, an overly-ambitious use of angiography provoke large ischaemic complications of the muscles and skin. In a retrospective case series analysed at a level I trauma centre it was found that complications provoked by angiography affected 11% of patients, and included gluteal muscle necrosis, surgical wound breakdown, deep infections, superficial infections, impotence and bladder necrosis [58].
Angiography also requires a specialised radiologist team and in most hospitals is probably not available at night or weekends. In any case, patients admitted at night or weekends for angiography and embolisation require longer for the technique to be performed and present a rate of mortality that is almost 100% higher than for patients admitted at other times [59]. In addition to the above techniques, new forms of intravascular management for bleeding during surgery are being proposed; thus, temporary partial intrailiac balloon occlusion during pelvis internal fixation is currently under study, but the results of this approach, although promising, are not yet definitively established [60].
2.2.2. Packing
Extraperitoneal packing, favoured by many European surgeons, is a safe and useful technique in cases of bleeding pelvic fractures, which provides an increase in systolic blood pressure (Fig. 14). Since packing requires laparotomy to enable an extraperitoneal approach without opening the peritoneal cavity, the repair of any source of abdominal or pelvic cavity bleeding is possible. Moreover, by approaching the retroperitoneum, injuries to major blood vessels can be treated immediately, including aorta artery clamping in the case of critical haemorrhage involving the iliac vessels [8]. The optimal role of each form of treatment for the management of bleeding pelvic fractures must be defined at each trauma centre, individually [61].
Packing is especially useful for “in extremis” patients unable to afford even a few minutes to undergo a CT-scan examination or when ring closure procedures fail. Despite the considerable importance of this technique, it is required only infrequently. In a series of over 600 pelvic trauma cases, only 18 patients underwent extraperitoneal packing. In this study, the 30-day survival rate after packing was 72%, and was correlated inversely with the patient’s age. However, this does not mean that the mortality rate after extraperitoneal packing is nearly 30%, because only one patient in this series died because of exsanguination. Further training in this technique is needed, as it is relatively infrequent. In a survey of more than 200 surgeons in Wales, although over 90% of the general surgeons responded that they would be able to perform a packing, and 84% would be prepared to cross-clamp the aorta, only 12% had undergone specific training for this, and only 45% had performed extraperitoneal packing (58% of the general surgeons and 34% of the orthopaedic surgeons) [62].
Controversy still exists about whether angiography or packing should be used as the first choice to address active bleeding. Studies of angiographic embolisation must take into account many cofounding variables, and the process takes much longer than for packing. Packing is a simpler technique for the trauma surgeon and is faster to perform than angiography [63]. In a retrospect review of a prospectively collected database of US academic level I trauma centres, comparing 20 angiography cases with another 20 treated by packing, it was found that the time elapsed from admission to packing averaged 45 minutes whereas for angiography the corresponding time was 130 minutes. Blood transfusion needs within 24 hours after admission were lower in the packing group, and mortality was higher in the angiography group [63]. Similar results were found in a level I Chinese centre [64]. The complementation of packing with angiography and embolisation appears to be a very reasonable strategy [65]. Some authors support the idea of conducting angiography and embolization for stable patients, but transferring unstable ones immediately to the operating theatre for packing; in the latter case, a subsequent postoperative embolisation might be performed if signs of further bleeding are observed [66]. In a systematic review, it was concluded that pelvic angiography plays a significant complementary role to pelvic packing for final haemorrhage control, and that pelvic packing, as part of a DC protocol, provides crucial extra time for a more selective management of the haemorrhage [67].
Since pelvic fracture is an important cause of major bleeding after trauma, replacement therapy appears to be essential. In Germany, the Pelvic Injury Registry set up a task force to examine how much fluid should be infused. On the question of the optimum volume of resuscitation fluid, unanimity has not yet been achieved; on the one hand, a low volume is not yet accepted in practice in the management of multiple trauma patients with pelvic fracture; on the other hand, a replacement therapy that is overly aggressive will contribute to haemodilution and subsequent coagulopathy [68].
Special attention must be paid in the case of open pelvic injuries. In a study of 29 battlefield trauma patients, the mean blood requirement during the first 24 hours was found to be 60.3 units. Ring closure in these patients was not possible, and in addition to the pelvic lesion, other injuries (vascular, bowel, genital and bladder) were often coexistent [69]. Such studies are of great importance, as the lessons learnt from treating combat injuries are subsequently transferred to the civilian context.
Once the patient is haemodynamically stable, thrombosis prophylaxis is mandatory. Mechanical thromboprophylaxis with intermittent pneumatic compression stocking should be instituted as early as possible (evidence grade 2C) [9], together with pharmacological thromboprophylaxis within 24 hours after bleeding has been controlled (evidence grade 1B) [9]. However, no consensus exists as to the best protocol for this purpose [70]. The use of low molecular weight heparin for a high risk embolism patient would be appropriate. However, vena cava filters in these patients, after massive bleeding, are not advisable (evidence grade 1C) [9].
3. FEMUR
Apart from pelvic trauma, fracture of the femur is the only fracture provoking acute life-threatening bleeding. Significant extremity injuries are associated with worse outcome, higher number of surgical procedures, greater need for blood transfusion and longer hospital stay [71].
Femur fractures are very easily diagnosed, as limb deformity clearly shows displacement of the femur. If possible, femur fractures in a high-energy severe trauma patient should be immobilised immediately, by external fixation (Fig. 15) [72, 73]. Should the patient be “in extremis”, and transferred directly to the operating theatre, with no time for external fixation, an external provisional femur fracture support, such as a sheet wrap around both extremities, should be applied. As stated previously, the use of a tourniquet to stop life-threatening bleeding from open extremity injuries is strongly recommended, and no account should be taken of tourniquet-induced pain. Nevertheless, the tourniquet should be released within two hours of application in order to avoid nerve paralysis and limb ischaemia (evidence grade 1B) [9]. Furthermore, some authors still consider skeletal traction to be useful as an external fixation technique for DC orthopaedics (DCO) [74].
It seems clear that femur fractures should be managed within 24 hours [75]; however whether this should be done with external fixation or with nailing remains unclear. A large study of nearly 500 femoral shaft fractures showed that DCO has a much shorter operative time than early total care, and produces less blood loss; nonetheless, no differences were observed between the two treatments with respect to respiratory distress syndrome, lung scores, multiple organ failure, intensive care unit stay or total hospital stay. Therefore, according to this study, the question of which fracture fixation method was adopted is not relevant to the incidence of systemic complications in severe trauma patients [76]. In another study, of over 1,300 cases, it was found that early intramedullary nailing was associated with a reduced need for mechanical ventilation and with lower hospital costs; this study addressed early versus delayed fixation [77]. The presence of concurrent lesions is, in any case, a very important variable for worsened outcome, particularly for abdominal injuries [73]. Bilateral femur fracture is also a predictor of increased mortality, but this is apparently associated with the simultaneous presence of more severe lesions and with poor physiologic parameters than with the presence of the bilaterality [78].
In summary, the clinical conditions for surgery must be taken into account in order to prevent complications. In a study of nearly 1,500 cases presenting fractures of the pelvis, acetabulum, spine or femur, a predictive model for complications was studied. Of these cases, 12% developed pulmonary complications and over 8% developed pneumonia. The ph and base excess were lower in these patients, as was the rate of improvement. Lactate was the most specific predictor of complications, with initial lactate being a stronger predictor of pneumonia than initial ph or the rate of improvement over the first 8 hours [75].
CONCLUSION
The concept of damage control (DC) is based on the idea of doing only what is indispensable to prevent the death of the patient or the development of major complications. This approach is now generally accepted to be the proper basis for clinical management of the severe trauma patient. DC orthopaedics for the treatment of haemodynamic instability, initially used in the case of femur fractures [19], has since been progressively extended to address pelvic fractures and is now being further extended to treat spinal fractures in order to avoid severe functional complications (Figs. 16-19) [79].
Massive transfusion adheres to the DC philosophy, and so the term “DC resuscitation” has been proposed for the coadjutant management of surgical resuscitation, consisting in performing a substantial infusion of blood products in order to treat hypovolemic shock and to prevent the self-perpetuating loop of coagulopathic haemorrhage, which can result in acidosis and hypothermia, the so-called “deadly triad”.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


