All published articles of this journal are available on ScienceDirect.
Efficacy of Percutaneous Vertebroplasty in the Treatment of Osteoporotic Vertebral Compression Fractures with Intravertebral Cleft
Abstract
Intravertebral cleft (IVC) is frequently observed in patients with painful osteoporotic vertebral compression fracture (OVCF). Some studies reported the usefulness of percutaneous vertebroplasty (PVP) for treating OVCF with IVC. However, systematic studies are scarce, and their results are conflicting. The purpose of this study was to evaluate the clinical and radiographic results of PVP in the treatment of painful OVCF with IVC. Two hundred ninety-one patients with OVCF with IVC underwent PVP. Back pain was measured using a visual analog scale (VAS), and physical disability was assessed using the Oswestry Disability Index (ODI). Three radiological parameters were assessed: the local kyphotic angle, percentage spinal canal cross-sectional area of compromise, and intravertebral instability of the affected vertebra. The mean follow-up period was 28 months. The mean values for the VAS and ODI were 8.4 and 60.0%, respectively, before PVP, versus 3.9 and 35.4%, respectively, at the final follow-up. The average local kyphotic angle, percentage spinal canal cross-sectional area of compromise, and intravertebral instability were 10.5°, 17.9% and 6.1°, respectively, before PVP and 8.1°, 15.2%, and 0.8°, respectively, at the final follow-up. There were no neurological or systemic complications due to cement leakage. PVP is an effective and safe intervention for treating OVCF with IVC.
INTRODUCTION
As the population ages, the incidence of osteoporotic vertebral compression fractures (OVCFs) has increased, representing a significant socio-economic problem. Instead of the generally believed good prognosis for most of these fractures, OVCF results in the long-term deterioration of patients’ health [1]. Percutaneous vertebroplasty (PVP) has been reported as an effective and less invasive technique for the treatment of painful OVCFs [2-5]. This cement augmentation technique is reported to produce excellent outcomes in >85% of treated patients [6].
On the contrary, intravertebral clefts (IVCs) have been reported in the imaging literature on OVCF. The initial reports dealt with air-filled clefts within vertebral compression fractures evident on conventional radiographs, and these findings were considered pathognomonic of ischemic necrosis [7-9]. Several studies described the magnetic resonance imaging (MRI) features of these clefts, which have a variable appearance depending on whether they are filled with gas or fluid at the time they are imaged [10-12]. Recently, the presence of an IVC within an osteoporotic vertebral fracture has been regarded as the manifestation of delayed traumatic collapse and pseudarthrosis [13-15], and osteoporotic vertebral fracture with IVC is linked with persistent pain [15-17].
Some studies reported the usefulness of PVP for treating OVCF with IVC. However, systematic studies are scarce, and their results are conflicting. Thus, an effective treatment for this state has not been established. The purposes of this study were to clarify the clinical and radiographic results of PVP in the treatment of painful OVCF with IVC and confirm the safety of PVP for treating OVCF with IVC.
MATERIALS AND METHODS
Patient Selection
Between 2003 and 2010, we enrolled patients who underwent single-level PVP for painful OVCF with IVC in our hospital. This study was approved by our institutional review board, and all patients received written informed consent for undergoing PVP prior to participation in this study.
Diagnosis
Painful osteoporotic vertebral pseudarthrosis was diagnosed by the presence of IVC and tenderness at the same level. All preoperative plain radiographic, computed tomography (CT), and MRI data were evaluated for diagnosing IVC. On plain radiography and CT, an IVC was defined as a linear well-demarcated focus of intervertebral fluid or gas attenuation [7]. On MRI, an IVC was defined as a linear well-demarcated focus of T2 prolongation similar to that of cerebrospinal fluid. Low signal intensity on T1- and T2-weighted images, which is characteristic of gas, was also considered to indicate an IVC [18, 19]. On dynamic contrast-enhanced MRI, IVC was illustrated as a noncontrast area, and vertebrae around the IVC were enhanced with contrast medium [20] (Fig. 1).
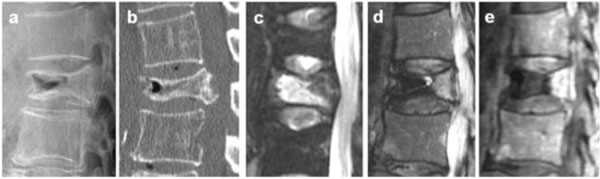
On radiographs and CT, IVC was defined as a linear well-demarcated focus of intervertebral fluid or gas attenuation (a: lateral radiographs, b: CT sagittal). On MRI, IVC was defined as a linear well-demarcated focus of T2 prolongation similar to that of adjacent cerebrospinal fluid. Signal void on T2- and T1-weighted images, which is characteristic of gas, was also considered an IVC (c: fat suppressed T2WI sagittal, d: T2WI sagittal). On dynamic contrast-enhanced MRI, IVC was shown as a non-contrast area and vertebra around the IVC was enhanced with contrast medium (e: sagittal).
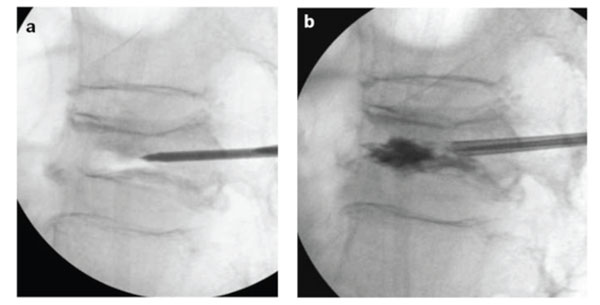
Before PMMA injection, we performed a “cavitygram” of the IVC using nonionic contrast agent to exclude needle placement within the basivertebral venous complex, and measured the capacity of IVC (a: before cavitygram, b: after cavitygram).
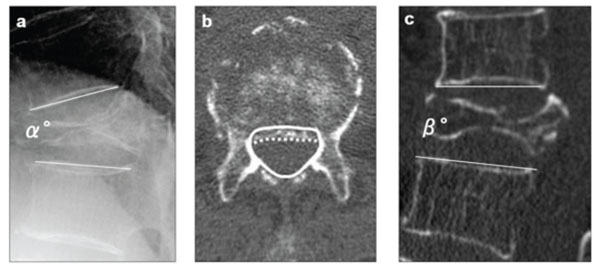
Radiographic parameters: (a) “local kyphotic angle” (α°) measured as the angle between the lower and upper endplates of the uninvolved vertebrae adjusted cephalic and caudal to the fractured vertebra on lateral radiography with the patient in a sitting position, (b) “percent spinal canal compromise” calculated by dividing the area of intrusion by total spinal canal area multiplied by 100. The total canal area is outlined by the solid line; the area of the retropulsed vertebral wall is demarcated by the dotted line. Areas of the spinal canal and retropulsed posterior wall are calculated from the total number of pixels per cross-section area (pixel/mm2), (c) intravertebral instability of the affected vertebra, measured as the difference between local kyphotic angle on lateral radiography with the patient in a sitting position (α°) and that on sagittal reformatted CT in a supine position (β°); α°-β°.

On CT before PVP, IVC was seen (a). On CT two years after PVP, bony bridge was seen between the fractured vertebra and the adjacent vertebra (b).
Inclusion and Exclusion Criteria
The selection criteria for PVP for treating OVCF with IVC were as follows: 1) sufficient back pain (visual analog scale [VAS] ≥ 4) refractory to standard medical treatment consisting of bed rest, analgesics, and/or external back bracing for at least 3 months; and 2) radiographic evidence of single-level OVCF with IVC with the presence of point tenderness on manual palpation. The exclusion criteria included the following: 1) spinal cancer, active infection, or uncorrectable bleeding diatheses; 2) inability to provide informed consent; and 3) a likelihood of noncompliance with direct follow-up; and 4) neurological deficit such as leg pain and motor weakness caused by neural compression or intravertebral instability of the affected vertebra.
PVP Procedure
PVP was performed by experienced spine surgeons. After general anesthesia, patients were carefully positioned in a prone position with extended posture on a radiolucent four-poster spinal frame. Next, 14-gauge bone needles (Ossiris; Hakko, Nagano, Japan) were inserted into the IVC through a bilateral transpedicular approach with direct biplane observation using a couple of fluoroscopes. The IVC was a confluent reservoir that was specifically targeted for polymethylmethacrylate (PMMA) (Osteobond; Zimmer, Warsaw, IN) injection. Before the PMMA injection, we performed a “cavitygram” of the IVC using nonionic contrast agent (Omnipaque 300; Nycomed, Princeton, NJ) to exclude needle placement within the basivertebral venous complex [21-23] and measured the capacity of the IVC via an injection of contrast medium from one side of the needle while the other side of the needle cannula was opened (Fig. 2). The residual contrast material was washed out with normal saline to clear the IVC adequately to visualize PMMA. Barium-opacified PMMA of the same volume as the capacity of the IVC was gently injected using 2-mL syringes. PMMA was injected via a one-sided needle with low pressure until the IVC was filled. PMMA assumed the shape of the IVC without evidence of extravasation into surrounding bone marrow space, paravertebral soft tissues, or epidural space. The procedure was terminated when the IVC was filled with PMMA. On the next day after PVP, CT was performed to determine whether extravertebral PMMA leakage had occurred. An orthopedic surgeon not involved in the procedure reviewed the CT images independently and reached a consensus for each case.
Radiographic Assessment
The following three radiographic parameters were assessed before PVP, 1 month after PVP and at final follow-up : 1) local kyphotic angle, measured as the angle between the lower and upper endplates of the uninvolved vertebrae adjusted cephalically and caudally to the fractured vertebra on lateral radiography with the patient in a sitting position; 2) percent spinal canal compromise on CT, calculated by dividing the area of intrusion by the total spinal canal area and multiplying the resulting value by 100; and 3) intravertebral instability of the affected vertebra, measured as the difference between the local kyphotic angle on lateral radiography with the patient in a sitting position and that on sagittal reconstructed CT with the patient in a supine position (Fig. 3).
Outcomes
Back pain was measured using a VAS score of 0-10, with 0 indicating no pain and 10 indicating the maximum imaginable pain [24]. Physical disability was measured using the Oswestry Disability Index (ODI) on a scale of 0-100%, with higher scores indicating greater disability [25]. The improvement rate was calculated using the following formula: ([baseline score − postoperative score]/baseline score) × 100. Patients were followed up directly and periodically after PVP. The mean follow-up period was 28 months (range, 6-55 months). Orthopedic surgeons not involved in the treatment performed the follow-up and clinical examinations to assess the patients’ functional status. The VAS score and ODI questionnaire o was self-administered and collected before PVP, 1 month after PVP and at final follow-up by a medical secretary to avoid interviewer bias.
Complications
Intra- and post-operative complications such as anesthesia- and general condition-related complications, surgical procedure-related complications, and cement leakage were evaluated. Risk factors for vertebral fractures adjacent to the augmented vertebra following PVP were also evaluated.
Statistical Analysis
Clinical characteristics, radiographic parameters, and procedural outcomes were analyzed using the Wilcoxon signed-rank test, Mann-Whitney U-test, and/or Bonferroni-Dunn post hoc test. The rates of procedure-related complications were estimated with 95% confidence intervals (CIs). Risk factors for vertebral fractures adjacent to the augmented vertebra following PVP were analyzed using multivariate logistic regression models. Statistical significance was defined at the level of p < 0.05 for a two-sided hypothesis. Data are presented as the mean ± standard deviation. Analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL).
RESULTS
Patient Sample
Two hundred ninety-one patients with painful OVCF with IVC (207 women and 84 men; mean age, 77 years) who underwent PVP at our institution were included in this study. Patients who died without operative complications (15), those with severe dementia (16), and those lost to follow-up (5) were excluded from the analysis, leaving 255 patients in this study. Patients were aged 61-93 years (mean 77) at the time of examination. OVCFs were detected from T7 to L5, and they occurred most frequently at the thoracolumbar junction (84%). There were 232 patients (91%) with primary osteoporosis and 23 patients (9%) with steroid-induced osteoporosis. The mean number of OVCFs was 1.2 in patients with primary osteoporosis and 1.4 in those with steroid-induced osteoporosis, and there were no significant differences in these findings. The mean duration from the onset of acute back pain to PVP was 29 ± 9.1 weeks (Table 1).
Baseline characteristics of the 255 patients.
| Characteristics parameters | N=255 |
| Age-yr | 77±7.1 |
| Female sex -no. (%) | 71 |
| Spinal level of OVCF with IVC - no. (%) | |
| T7-T10 | 13 |
| T11 | 16 |
| T12 | 83 |
| L1 | 89 |
| L2 | 29 |
| L3-L5 | 25 |
| Duration from OVCF to PVP - week | 29±9.1 |
| VAS score for back pain | 8.4±1.6 |
| ODI (%) | 60.0±17.4 |
Radiographic parameters before PVP, one month after PVP and at final follow-up.
| Before PVP | One Month After PVP |
Final Follow-Up |
|
|---|---|---|---|
| Local kyphotic angle | 10.5 ° | 6.2 °* | 8.1 °* |
| Percentage spinal canal cross-sectional area of compromise |
7.9 % | 17.1 % | 15.2 % |
| Intravertebral instability | 6.1 ° | 1.7 °* | 0.8 °* |
* p<0.001 versus before PVP.
Back pain (VAS) and physical disability (ODI).
| Before PVP |
One Month After PVP |
Final Follow-Up |
|
|---|---|---|---|
| VAS (mean±SD) improvement rates |
8.4±1.6 | 2.3±2.1 * 72.6% |
3.9±3.0 * 53.6% |
| ODI (mean±SD) improvement rates |
60.0±17.4% | 33.1±20.2% * 44.8% |
35.4±12.6% * 41.0% |
| VAS 0 (%) VAS 1~3 (%) |
0 0 |
28.6 45.1 |
18.0 40.0 |
* p<0.001 versus before PVP.
The rate of incidence of cement leakage.
| Cement Leakage Occurrence (%) |
95% CI | |
|---|---|---|
| Total incidence of cement leakage | 60 (23.5%) | 18.5-29.2 |
| Epidural space | 3 (1.2%) | 0.2-3.4 |
| Perivertebral soft tissue | 12 (4.7%) | 2.5-8.1 |
| Intervertebral disc space | 45 (17%) | 13.2-22.9 |
Prognostic factors for vertebral fracture adjacent to the augmented vertebra. Analysis with a multivariate logistic regression model.
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Age | 2.0 (1.2-3.4) | 0.013 |
| Sex | 1.3(0.3-5.3) | 0.690 |
| Bone density | 0.1(0.0-5.9) | 0.280 |
| Injected PMMA volume | 1.1 (1.0-1.3) | 0.007 |
| Local kyphotic angle before surgery | 1.1 (1.0-1.2) | 0.017 |
| PMMA leakage to the disc space | 4.1(0.1-112.7) | 0.393 |
Radiographic Assessment
The average local kyphotic angle was 10.5° before PVP, 6.2° 1 month after PVP, and 8.1° at the final follow-up. The average local kyphotic angles 1 month after PVP and at the final follow-up were significantly smaller than that before PVP (p < 0.001). The average percentage spinal canal cross-sectional area of compromise was 7.9% before PVP, 17.1% 1 month after PVP, and 15.2% at the final follow-up. There was no statistical significance in the average percentage spinal canal cross-sectional area among the time points. The average intravertebral instability was 6.1° before PVP, 1.7° 1 month after PVP, and 0.8° at the final follow-up. The average intravertebral instability 1 month after PVP and that at the final follow-up was significantly less than that before PVP (p < 0.001) (Table 2). In total, 62 of 106 (58.5%) patients who received the reconstructed CT 2 years after PVP exhibited bony bridges between the fractured vertebra and the lower or upper vertebrae on sagittal reconstructed CT 2 years after PVP (Fig. 4).
Outcomes
The mean VAS values were 8.4 before PVP, 2.3 1 month after PVP, and 3.9 at the final follow-up. The improvement rates according to the VAS were 72.6% 1 month after PVP and 53.6% at the final follow-up. All patients displayed statistically significant improvements in the VAS after PVP (p < 0.001). The mean ODI values were 60.0% before PVP, 33.1% 1 month after PVP, and 35.4% at the final follow-up. The recovery rates according to the ODI were 45.1% 1 month after PVP and 40.0% at the final follow-up. All patients exhibited statistically significant improvement in the ODI after PVP (p < 0.001). The numbers of patients with no back pain (VAS = 0) were 73 (28.6%) 1 month after PVP and 46 (18%) at the final follow-up (Table 3).
Complications
Cement leakage was observed in 60 of 255 patients (23.5%). Small amounts of cement leakage occurred in the epidural space (3 of 255 patients, 1.2%), perivertebral soft tissue (12 of 255, 4.7%), and intervertebral disc space (45 of 255, 17%) (Table 4). There were no neurological or systemic complications due to cement leakage. Vertebral fracture adjacent to the augmented vertebra was noted in 62 of 255 patients (24.3%). The risk factors for vertebral fracture adjacent to the augmented vertebra were evaluated. We analyzed age, sex, bone density, injected PMMA volume, local kyphotic angle before surgery, and PMMA leakage into the disc space as risk factors for vertebral fracture. The risk factors for new fracture were the injected PMMA volume (odds ratio [OR] = 1.1, 95% CI = 1.0-1.3, p = 0.007), local kyphotic angle before surgery (OR = 1.1, 95% CI = 1.0-1.2, p = 0.017), and age (OR = 2.0, 95% CI = 1.2-3.4, p = 0.013) (Table 5).
DISCUSSION
This study demonstrated that PVP is an effective and safe intervention for the treatment of OVCF with IVC. The most important aspect of this procedure was the injection of PMMA into the IVC with the assistance of a cavitygram, ensuring that no additional pressure was exerted.
OVCF has been considered to display a benign natural history. However, rigorous follow-up studies have clarified that OVCF does not respond adequately to standard conservative therapy in up to 30% of patients [26-28], and the condition is associated with the long-term deterioration of patient health [1]. Several reports noted that the presence of OVCF with IVC is associated with a poor prognosis and that the condition could prolong low back pain [15-17]. No definitive surgical options are available for the treatment of OVCF with IVC and long-lasting severe back pain or delayed neurologic deficits. A combined anterior and posterior procedure may maximize the likelihood for successful fusion, particularly with multiple points of spinal fixation and occasionally with PMMA augmentation [26]. However, large surgical interventions remain challenging for patients of advanced age, those with medical co-morbidities, and those with poor fixation secondary to osteoporosis [29].
In performing PVP for treating OVCF, there are some risks of cement leakage [30-34]. Nieuwenhuijse et al. [30] and Ha et al. [31] reported high rates of cement leakage in vertebroplasty for OVCF with IVC. They injected PMMA with high pressure and filled the intravertebral cancellous bone with the agent. On the contrary, Tanigawa et al. [32] reported no statistically significant difference in the incidence of cement leakage for OVCF with or without a cleft. In our series, bone needles were inserted into the IVC through a bilateral transpedicular approach, and the IVC was specifically targeted for PMMA injection. Furthermore, a cavitygram of the IVC was performed [21-23] to measure the capacity of the IVC to decrease the risk of cement leakage. PMMA was injected from the one-sided needle with low pressure until the IVC was filled. In our series, cement leakage was observed in 56 of 244 patients (23.0%). This rate of cement leakage appears low compared with that reported previously [30-32]. We believe that PMMA injection into the IVC after a cavitygram might reduce the risk of cement leakage.
One may consider PMMA injection into the IVC alone insufficient for maintaining the spinal alignment, and this strategy might increase the risk of kyphosis after PVP. It is true that PVP does not improve global spinal alignment and PVP is not indicated for the patients with pain affected by the global imbalance of spine. For those patients, spinal reconstruction surgery with instrumentation is recommended. In our series, the average local kyphotic angles 1 month after PVP and at the final follow-up were significantly smaller than that before PVP. This finding indicated that the spinal alignment is preserved to some degree after PVP. Moreover, the average intravertebral instability 1 month after PVP and at the final follow-up was significantly less than that before PVP. We believe that the clinical symptoms (VAS and ODI) were correlated with the intravertebral instability. McKiernan et al. [35] firstly reported that intravertebral instability is substantial and clinically significant in the treatment of OVCF. Hoshino et al. [36] indicated that intravertebral instability affected the severity of back pain. It is important to alleviate the intravertebral instability by injecting PMMA into the IVC in the treatment of OVCF.
In conclusion, the current study identified PVP as an effective and safe intervention in the treatment of OVCF with IVC, and vertebroplasty could be readily performed with the injection of an amount of PMMA equal to the capacity of IVC to stabilize the affected vertebra. There were favorable outcomes and no neurological or systemic complications due to cement leakage. Based on our results, we believe that PVP could be an alternative method for the treatment of OVCF with IVC.
ABBREVIATIONS
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


