All published articles of this journal are available on ScienceDirect.
Concomitant Septic Arthritis and Tophaceous Gout of the Knee Managed with Intermittent Closed Joint Irrigation Combined with Negative Pressure Therapy: A Case Study and Literature Review
Abstract
Tophaceous gout complicated by septic arthritis presents a management dilemma which can often require multiple surgical debridements. There is little published in the literature regarding treatment of these concomitant conditions. We postulate that biofilm may play a role increasing the difficulty of sterilising a tophaceous joint. The use of topical negative pressure therapy that targets biofilm has been well established for a range of wounds. A new device that incorporates both intermittent negative pressure therapy and wound irrigation was introduced in 2012. This case report describes the use of this topical negative device with the instillation option in the management of severe septic arthritis with concomitant gout and suggests directions for further research.
INTRODUCTION
Gout is characterised by deposition of monosodium urate crystals in tissues with a subsequent chronic inflammatory response. Prevalence of tophaceous gout has been observed to decrease due to advancements in medical management of gout and subsequent reduced rates of hyperuricaemia [1] however, gout still affects approximately 1% of the population [2].
The incidence of septic arthritis complicating crystal arthropathy in patients presenting with monoarthritis has been reported to be between 1.5% [3] and 5.2% [4]. The presence of crystals alone cannot exclude infection [3-7], and high synovial fluid WBC counts and elevated CRP are common in both conditions [4] so a high index of suspicion on concomitant pathologies is required [6].
Literature regarding concomitant gout and septic arthritis is largely limited to case reports [3-12] and little has been published regarding management. Management options reported in the literature have included medical management, surgical debridement, arthrodesis and amputation [6, 7]. Duan et al. [9] reported debridement in addition to the use of a negative pressure drainage device.
Patients with long term tophaceous gout are vulnerable to infection of the tophi. Medical management of tophaceous gout is first line, however indications for surgical management include restoration of function, to provide symptomatic relief, to manage infection and to decrease total body urate loading [1, 13]. Kumar et al. [14] and Lee et al. [1] observed that the group most likely to proceed to operative management of gouty tophi was those who presented with sepsis (infected/ulcerated tophi). Kumar et al. [14] also found that this group was also most likely to experience delayed wound healing. There is little reported data regarding management, medical or surgical, of patients with septic arthritis complicating gout.
In our case study of a patient presenting with concomitant tophaceous gout and septic arthritis, we illustrate the management difficulty posed by this clinical scenario and postulate reasons for this. We propose that biofilm may play a role in gout; a concept that has not been identified in literature to date, and therefore suggest that management strategies that target biofilm specifically could be utilised.
CASE STUDY
The patient, aged 54, presented to a major tertiary hospital in 2012 with a history of increasing pain in the left knee over the previous three days. On examination he was febrile, tachycardic and the knee was erythematous and swollen. He was unable to bear weight and flexion of the left knee was limited to a range of 5-40 degrees. This presentation was two weeks after arthroscopy of the left knee. He had a history of severe poliarticular tophaceous gout that had led to widespread joint damage.
The onset of gout was at age 17 and had been propagated by a long history of high alcohol consumption. Joints affected by the gout included the knees, elbows, ankles and small joints of the hands (see Figs. 1, 2). In the past he had arthroscopies of elbows, right knee and right ankle for resection of gouty tophi and was on long term prednisolone (5-25 mg daily) and allopurinol (300 mg daily). He had no history of septic arthritis. Other medical background included hypertension and obesity.
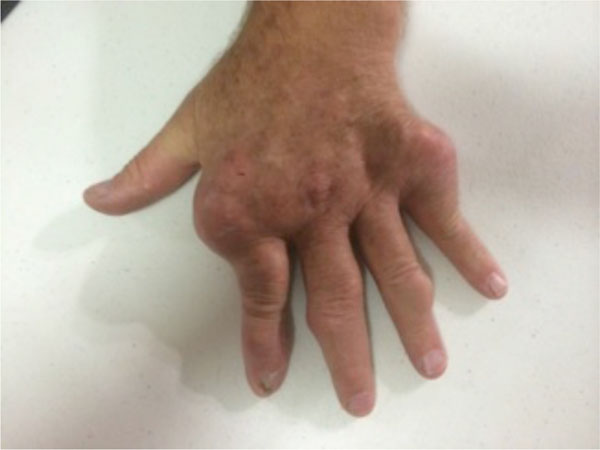
Gout affecting the joints of the left hand.
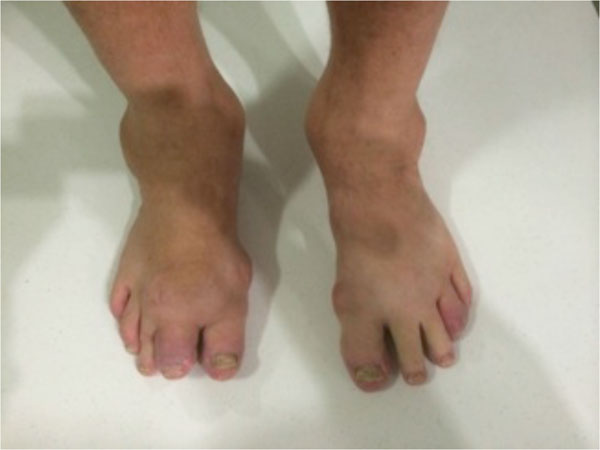
Joints of the patient’s feet demonstrate gouty tophi.
C-reactive protein (CRP) was 260 mg/L and knee aspirate revealed thick gritty purulent exudate. A diagnosis was made of left knee joint septic arthritis on a background of severe poliarticular tophaceous gout and immunocompromise secondary to regular prednisolone.
IV Vancomycin and Flucloxacillin were commenced and he underwent emergent arthroscopic washout in theatre on the day of admission. The chalky, gritty accumulations of monosodium urate crystals were expressed followed by a thorough washout (see Figs. 3, 4).
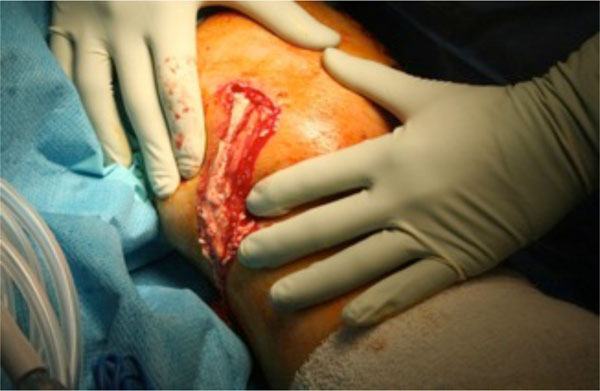
Exudate expressed from left knee at initial arthroscopic washout.
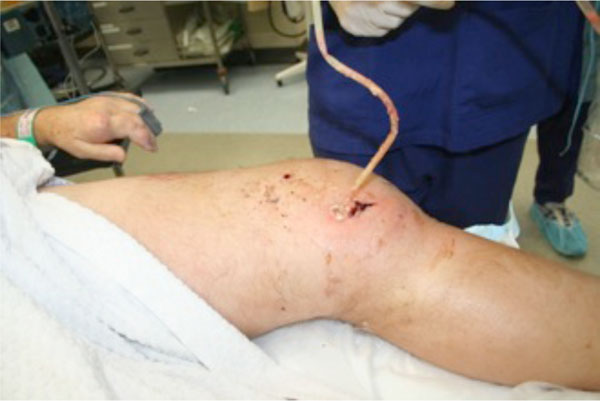
Debridement and washout of the left knee.
Minimal clinical improvement was observed following the arthroscopic washout so open washout was undertaken the following day. This was followed by admission to the high dependency unit with overt sepsis.
Both the initial aspirate and the synovial tissue specimens collected intra-operatively during the arthroscopic washout demonstrated urate crystals and cultured enterococcus faecalis and streptococcus viridians. Both organisms were sensitive to Amoxicillin and Vancomycin and so antibiotic therapy was rationalised to IV Amoxicillin. Allopurinol was increased to 300 mg twice daily to address the component of inflammation driven by gout.
Despite these measures, increasing pain and erythema precipitated a repeat open washout nine days later. Wound closure was not possible due to marked swelling and a Topical Negative Pressure dressing using V.A.C® technology was applied. Amoxicillin was changed to Piperacillin with Tazobactam (Tazocin) and Vancomycin as he was not responding to the Amoxicillin and had further septic deterioration following this washout.
After two weeks of microbiologically-appropriate IV antibiotics and multiple further surgical washouts, the patient remained very unwell with evidence of ongoing sepsis of the knee and nothing to suggest an alternative source. At each subsequent washout samples sent for microscopy, culture and sensitivity demonstrated growth of enterococcus faecalis alone which remained sensitive to both Amoxicillin and Vancomycin. There were discussions that an above knee amputation may be required as a life-saving intervention if the sepsis could not be brought under control.
Given that all previous debridement and washouts were quickly followed by re-accumulation of infected tophaceous material in the knee, a decision was made to try an intermittent infusion of an antiseptic solution. On day 26 of admission, a further joint debridement and washout was undertaken and three intra-articular portals were established based on previous incisions (see Fig. 5).
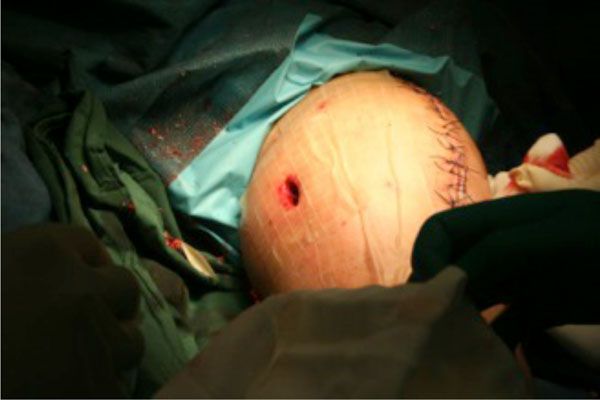
Intra-articular portals to enable access to the joint space.
These portals were used in conjunction with a Topical Negative Pressure Wound Therapy (NPWT) system which allowed regulated closed infusion and soaking with an antiseptic solution [15] (see Fig. 6).

V.A.C. VeraFlo™ negative pressure equipment.
In the absence of any prior literature for this specific application we elected to use Prontosan®, an antiseptic irrigation solution containing 99.8% purified water, 0.1% Betaine ( surfactant) and 0.1% Polyaminopropyl Biguanide (Polyhexanide) as a preservative also known for its antimicrobial properties. This was selected on reports that it is non-irritant and helps to remove biofilm [16]. There is no reported resistance to its components, it has minimal cell toxicity and does not inhibit granulation and epithelialisation [16]. Whilst we could not identify previous reports for its use in septic arthritis, control of bacterial burden has been objectively demonstrated with skin wounds including chronic infected venous ulcers [15].
Programming the infusion of the soak is flexible for both duration and frequency. In this case the device was programmed to deliver four hourly instillations of 20 ml of Prontosan® directly into the joint space via the three portals. This was set at a soak time of 30 minutes before conventional topical negative pressure was recommenced at the standard 125 mmHg negative pressure. A continuous passive movement device was used for two hours three times a day to achieve knee flexion starting initially at 0- 40 degrees with gradual increments aimed to achieve 0- 90 degrees
The clinical picture improved rapidly with reduced frequency of febrile episodes and the patient reported a marked improvement in knee pain. Allopurinol was increased to 900 mg daily at the recommendation of the rheumatology team. The reduction of CRP following the infusion therapy is illustrated (Graph 1).
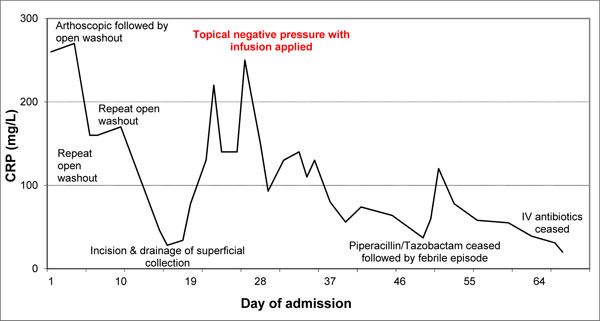
C-reactive protein (CRP) trend with surgical intervention.
Tazocin IV was ceased three weeks after the attachment of the topical negative pressure device but this was followed by a further febrile episode associated with increasing inflammatory markers. Tazocin was recommenced with immediate effect and there were no further febrile episodes.
By day 53 of admission, four weeks after attachment of topical negative pressure device, the extent of healing of the three incisions meant that the instillations were no longer penetrating to the joint space (see Fig. 7). The instillation therapy was ceased and the incisions continued healing via secondary intention. The Vancomycin and Tazocin were continued for a total of six weeks, followed by a three-month course of oral Amoxicillin.
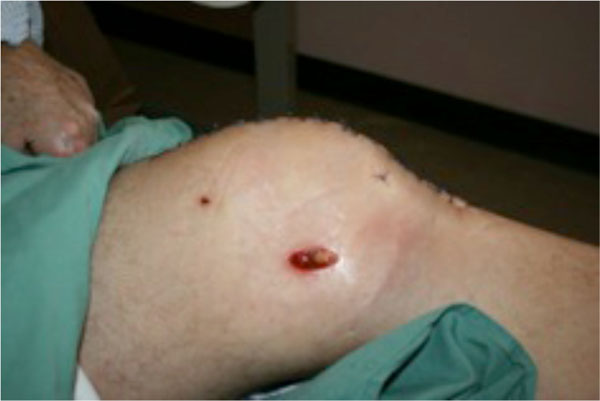
Left knee after V.A.C. VeraFlo™ therapy.
On discharge, range of movement had greatly improved with flexion to 90 degrees and he mobilised with one stick. 18 months post discharge he was able to mobilise independently (see Figs. 8, 9).
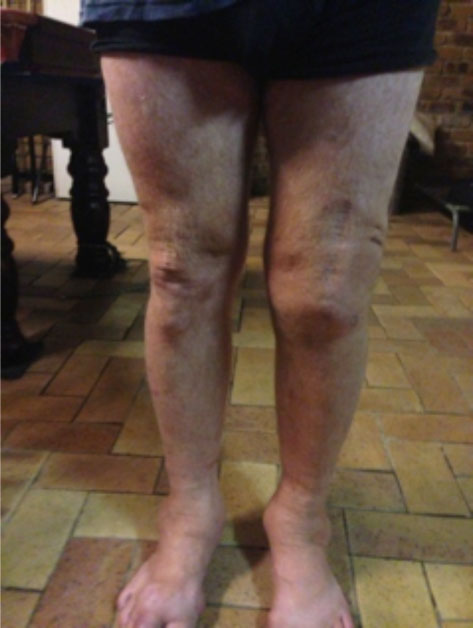
Able to mobilise independently18 months post discharge.
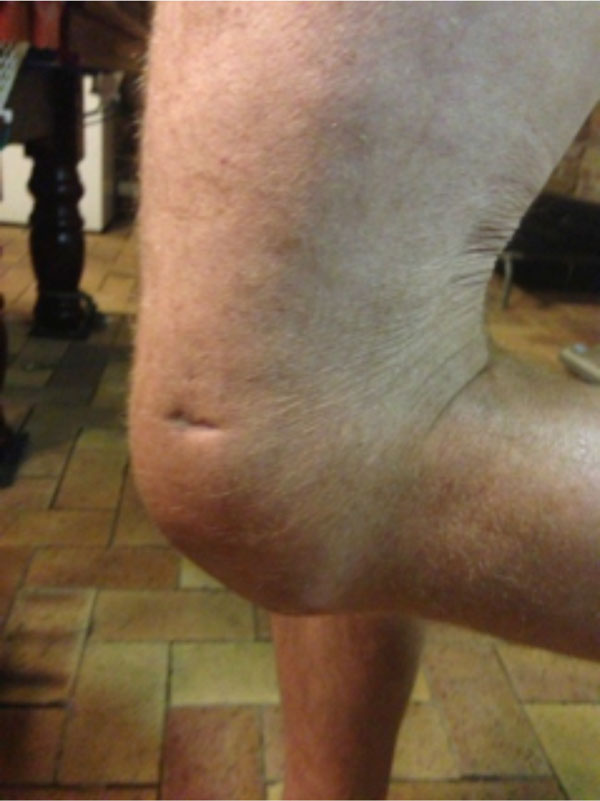
Flexion of knee to 90 degrees 18 months post discharge.
In summary, the patient presented with concomitant septic arthritis and severe poliarticular tophaeous gout affecting his knee. This rapidly progressed to overt sepsis requiring admission to the high dependency unit, six operative debridement and washouts and an extended course of broad spectrum intravenous antibiotics. It appeared that sepsis control was not obtained until the introduction of the intermittent closed system joint infusions. Following this no further knee washouts were required.
DISCUSSION
This case of concomitant gout and septic arthritis did not appear to respond to conventional management of repeat washouts, debridement and broad spectrum IV antibiotics. This raises the question of what makes this type of presentation so difficult to manage.
The composition of gouty tophi is precipitated monosodium urate crystals in a largely avascular environment, therefore theoretically isolated from effective host immune responses in the context of infection. The limited literature regarding the in vivo immune response to gouty tophi suggests that T-lymphoctyes, macrophages and foreign body giant cells are involved, with B-lymphocytes, plasma cells and neutrophils also present to a lesser degree [17]. This knowledge however is yet to be translated into management principles for management of tophaceous gout.
When considering the pathophysiology of resistant infections in an orthopaedic context, the issue of biofilm would be considered. Biofilms are surface-associated communities of bacteria surrounded in an extracellular matrix [18]. This provides additional protection for pathogens via alterations to the extracellular matrix production and cell-to-cell adhesion, and heavy bacterial bioburden increases the metabolic requirements, stimulates pro-inflammatory mediators and can negatively impact on wound healing [19]. Gabriel et al. [20] suggest that these bacteria also secrete harmful cytokines which can lead to vasoconstriction and decreased blood flow. These structures enable bacteria to survive at much higher concentrations of antibiotics [18]. It has been well documented that enterococcus faecalis forms biofilm [18, 21].This organism was initially identifiedand remained persistent throughout this case inspite of appropriate sensitivity directedantibiotic use. Current literature on biofilm indicates that for our patient, the two week period between initial arthroscopy and presentation with acute monoarthopathy would be adequate for biofilm to form: bacteria may form biofilm within five hours following bacterial proliferation and mature biofilm can be observed within eight to ten hours [19].
In the setting of gout however, the role of biofilm in treatment-resistant infection would be extrapolation of existing knowledge on biofilm, as no literature was identified that addresses the role of biofilm in gouty arthritis.
With the assumption that biofilm had a role in this case study, this raises the possibility of the use of management techniques that specifically target biofilm. Cowan et al. [22] describe the results of both in vitro and in vivo animal experiments with pig wounds with mature biofilms which has led to the concept of “biofilm-based wound care”, which combines both surgical debridement “followed by topical therapies that kill residual biofilm micro colonies and prevent the reformation of biofilm communities” [22]. Willy [23] provided a comprehensive update on the role of topical antiseptics, emulsifiers and mechanical energy as options to eradicate bacterial burden in wounds.
The use of wound irrigation is a long standing principle used for contaminated wounds. This concept was popularised in 2003 and was made commercially available with an intermittent instillation option used in conjunction with negative pressure wound therapy (V.A.C. Instill Wound Therapy System, Kinetic Concepts Inc. San Antonio, TX). It was not until 2012 when the next generation of NPWT devices with instillation was introduced that this mode of therapy gained popularity. This instillation technology uses reticulated open-cell foam dressings that are less hydrophobic and specifically designed for instillation therapy. Currently, only anecdotal evidence is available that suggests efficacy.
Recently the use of standard NPWT provides an option to include frequent, periodic, instillation of solutions into the wound bed. Results from in vitro models of NPWT treatment of mature biofilms grown on pig skin implants show that NPWT with instillation of various antiseptic solutions containing topical antiseptics such as dilute iodine, Polyhexanide, CHG, sodium hyperchlorite, or large polyquat polymers significantly reduced biofilm levels in 24 hours [23].
The effect of this therapy may not be due to the antiseptic therapy role alone. It has been postulated that the negative pressure has a mechanical effect whereby it creates a strain on the biofilm and bacterial cell membranes which facilitates their breakdown. Thus it is the combination of soaking and sustained NPWT between soaks that may be providing the effect and is worthy of further studies to test this hypothesis and determine ideal combinations of fluids, soak times and negative pressure applications [24].
Current literature regarding biofilm addresses the pathophysiology and management of surface wounds whereas in this case involved an intra-articular pathology. This has not been previously studied in the context of fluid instillation and NPWT.
This case illustrates the management difficulty posed by concomitant gout and septic arthritis. In spite of appropriate antibiotic use, infection continued until a strategy known to act on biofilm was instituted. We postulate that biofilm may form on avascular gouty tophi. The monosodium urate precipitate may be analogous to medical implants in the setting of infection and therefore as difficult to sterilise due to the biofilm principles of attachment and cell to cell adhesion demonstrated in implants [19]. Management approaches that target biofilm may merit further study if biofilm is demonstrated to play a role. If this is the case, the combination of an appropriate soaking solution with NPWT in a closed system after initial arthroscopic or open debridement may be a reasonable approach to manage a septic joint with gouty tophi not responding to initial debridement and washout.


