All published articles of this journal are available on ScienceDirect.
The Pathophysiology, Diagnosis and Current Management of Acute Compartment Syndrome
Abstract
Acute compartment syndrome (ACS) is a surgical emergency warranting prompt evaluation and treatment. It can occur with any elevation in interstitial pressure in a closed osseo-fascial compartment. Resultant ischaemic damage may be irreversible within six hours and can result in long-term morbidity and even death. The diagnosis is largely clinical with the classical description of ‘pain out of proportion to the injury’. Compartment pressure monitors can be a helpful adjunct where the diagnosis is in doubt. Initial treatment is with the removal of any constricting dressings or casts, avoiding hypotension and optimizing tissue perfusion by keeping the limb at heart level. If symptoms persist, definitive treatment is necessary with timely surgical decompression of all the involved compartments. This article reviews the pathophysiology, diagnosis and current management of ACS.
INTRODUCTION
Richard von Volkmann first described compartment syndrome in 1881 [1]. He suggested that paralysis and contracture came simultaneously as a result of an interruption to the blood supply of the affected muscles. The first surgeon to reproduce ischaemic contracture in animals was Paul Jepson in 1924 [2] whilst working at the Mayo Foundation. He also demonstrated that prompt surgical decompression could prevent these contractures. Acute compartment syndrome (ACS) is now considered a surgical emergency warranting prompt evaluation and treatment.
PATHOPHYSIOLOGY
ACS is defined as ‘a critical pressure increase within a confined compartmental space causing a decline in the perfusion pressure to the tissue within that compartment’ [3-6]. It can occur with any elevation in interstitial pressure within an osseo-fascial compartment. Tissue perfusion is proportional to the difference between capillary perfusion pressure (CPP) and the interstitial fluid pressure.
When fluid enters a fixed volume compartment, for example from bleeding, both the tissue and venous pressure increase. When this exceesds the CPP, capillary collapse with ensuing muscle and nerve ischaemia occur. A similar reduction occurs in the CPP when the compartment size decreases (e.g. external compression) due to an increase in intracompartmental pressure, as well as a reduction in the arteriolar pressure. Fig. (1) below displays the cycle of events and the development of acute compartment syndrome.
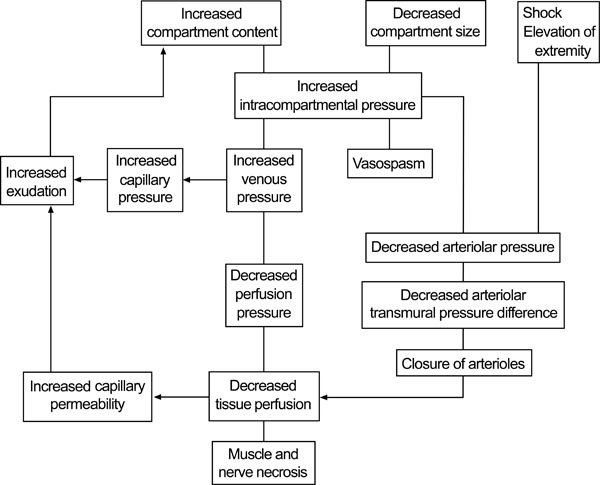
Pathophysiology of compartment syndrome. © 2011 American Academy of Orthopaedic Surgeons. Reprinted from the Journal of the American Academy of Orthopaedic Surgeons, Volume 19 [1], pp. 49-58 with permission.
Compartment syndromes can arise in any area of the body that has little or no capacity for tissue expansion.
The commonest cause of all ACSs are tibial shaft fractures with a range from 2-9% [7, 8]. After the leg, the next commonest location is in the forearm, but almost any compartment can be affected: arm [9], thigh [10], foot [11], buttock [12], hand [13], and abdomen [14]. Poor patient positioning in unconscious patients for long periods of time can also contribute to the aetiology of ACS [15].
Any internal or external event that increases intra-compartmental pressure can cause a compartment syndrome. Table 1 below shows some of the more common causes.
Common causes of ACS.
| Fracture | Burns |
| Crush injury | Infection |
| Injection injury | Bleeding disorders |
| Penetrating trauma | Arterial injury |
| Constrictive dressings | Reperfusion |
| Casts | Extravasation of drugs |
The incidence is thought to be 3.1 per 100000 population, with males ten times more commonly affected than females [16, 17].
OUTCOME
Prognosis is dependent on a number of factors:
- Injury severity.
- Duration of ischaemia.
- Pre-injury status and comorbidities.
- And most importantly time to fasciotomy.
Rorabeck concluded ‘almost complete recovery of limb function if fasciotomy was performed within six hours' [18]. When fasciotomy was performed within twelve hours normal limb function was regained in only 68% of patients; after twelve hours only 8% regained normal function [19].
With late diagnosis, irreversible tissue ischaemia develops causing potentially disastrous neurological deficits, muscle necrosis, ischaemic contracture, infection, chronic pain, delayed fracture union [20], rhabdomyolysis [21], amputation, and even death.
DIAGNOSIS
ACS is a clinical diagnosis; the most important determinant of outcome is early recognition and expeditious surgical intervention [22, 23].
Classically the five Ps (pain, pallor, pulselessness, paralysis and paraesthesia) are taught as the symptoms heralding a compartment syndrome. Pain is usually ‘out of proportion’ to the injury requiring increasing doses of strong opiates. The pain is often described as burning, deep in nature and is reproduced with passive stretching of the muscles in that compartment. In severe trauma or when the patient is unconscious pain may, however, be difficult to assess. Pain is also subjective and has a poor sensitivity [24].
Pulselessness and paralysis are rare, only occurring after an arterial injury or after a substantial amount of time has elapsed. Clues may also be evident from the history: high-energy injuries, anticoagulation therapy or haemophilia significantly increase the likelihood of developing ACS.
Physical signs are often few but there may be a firm, wooden feeling on deep palpation. Reduced two-point discrimination or vibration sense may be found in the early stages; if a major sensory deficit is evident the syndrome is already far advanced.
‘Pain out of proportion to the injury’ and ‘pain with passive stretching of the muscles in the compartment’ are the earliest, most reliable indicators of ACS [25].
If the diagnosis is not in doubt, emergent surgical fasciotomy is needed. Where doubt remains, the intra-compartmental pressures can be measured. A transducer connected to a catheter is introduced under aseptic conditions into the compartment in question within 5 cm of the zone of injury. Proprietary devices are available (e.g. Stryker monitor [26]) or a transducer can be fashioned from standard hospital equipment [27] (Fig. 2a-2b). The anterior compartment is the most affected and accessible in the leg and often compartment pressures are measured here first. All compartments in the affected limb should be measured if there is enough clinical concern [28]; marginal readings should be repeated with further compartment pressure readings and repeat physical exam.

Stryker compartment pressure measurement device.
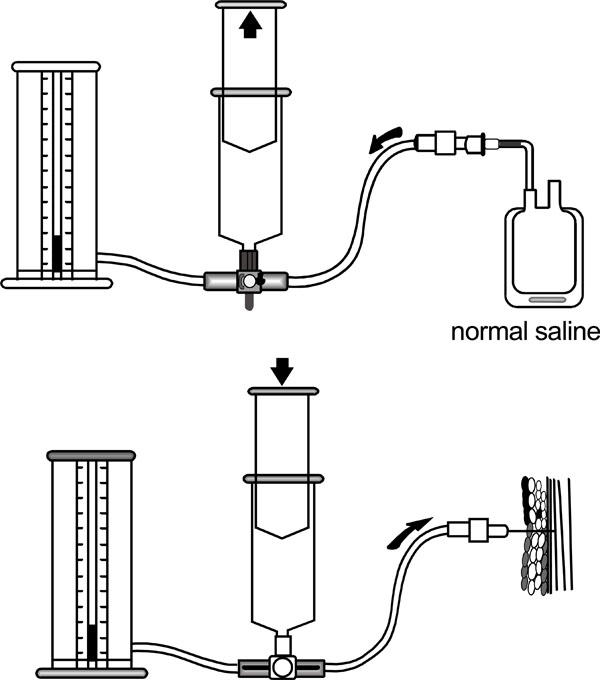
Pressure transducer using a normal saline injection and standard manometer.
Many authors advocate the use of continuous intra-compartmental pressure monitoring in all at risk patients [29] and those where extra clinical vigilance is advised [30]. It has also been suggested that pressure monitoring may ‘detect ACS prior to the onset of clinical signs, in addition to reducing the time to fasciotomy and the development of subsequent sequelae’ [31], ‘whilst not increasing the rate of fasciotomy or associated complications’ [32].
The normal intra-compartmental pressure of healthy muscle is roughly 10 mmHg [33]. Initial studies showed absolute intra-compartmental pressure values of 30 mmHg, 45 mmHg and 50 mmHg as the critical threshold above which circulation is compromised. The lower level of 30 mmHg has been more commonly quoted as animal studies have shown that muscle blood flow cannot be maintained and the fascia is maximally stretched [34].
Whitesides et al. [35] subsequently introduced the concept that the threshold at which irreversible damage was done is variable and dependent on the perfusion pressure.
This takes into account the variability of the patient’s blood pressure maintaining (or not) adequate tissue perfusion. The pressure difference or ‘delta pressure’ is the diastolic blood pressure minus intra-compartmental pressure. McQueen and Court-Brown [36] prospectively studied 116 patients with diaphyseal tibial fractures and concluded a threshold delta pressure for decompression of 30 mmHg led to ‘no missed cases, unnecessary fasciotomies or significant complications’ of ACS. White et al. studied 101 patients and validated a delta pressure of 30 mmHg or less [37].
TREATMENT
Immediate management involves the identification and removal of external compressive forces, and releasing casts or dressings down to the skin. The limb should not be elevated and instead kept at the level of the heart so as not to decrease arterial flow any further [38].
Early assessment of hypovolaemia, metabolic acidosis and myoglobinaemia is mandatory to avoid potential renal failure. Intravenous fluids and supplemental oxygen may be needed, as well as regular blood biochemistry and urinalysis. It is important to maintain normotension as hypotension may decrease perfusion further and compound any existing tissue injury [39].
If the clinical features of ACS do not improve following simple measures, definitive surgical fasciotomy is required on an emergency basis. In conjunction with fasciotomy, orthopaedic, vascular and plastic surgery input may often be necessary to deal with concomitant injuries. Primary amputation can be considered if the diagnosis is delayed, there is no muscle function and there has been significant trauma to that limb.
The principles of fasciotomy include:
- Adequate and extensile incision
- Complete release of all involved compartment
- Preservation of vital structure
- Thorough debridement
- Skin coverage at a later date (7-10 days)
Post-operative pain is a major feature of ACS and adequate analgesia should be prescribed on a regular basis. The patient should be monitored closely for potential complications, in particular rhabdomyolysis and acute renal failure. An adequate urine output of >0.5 mL/kg should be maintained with additional intravenous fluid administration. Mannitol has been used in the past as an adjunct in an attempt to lower intra-compartmental pressure, but may be more helpful in ischaemic-reperfusion injuries [40].
LEG
The leg consists of four anatomical compartments (Fig. 3):
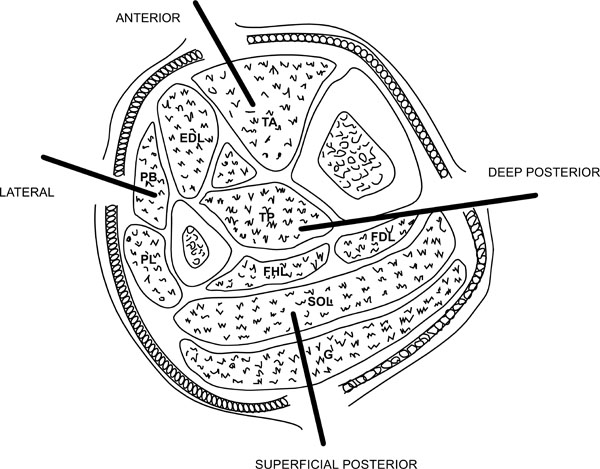
The compartments of the leg.
- Anterior compartment containing tibialis anterior, extensor digitorum longus, extensor hallucis longus and peroneus tertius.
- Posterior superficial containing the gastrosoleus complex.
- Lateral compartment containing peroneus longus and brevis.
Posterior superficial containing the gastrosoleus complex. - Posterior deep compartment containing tibialis posterior, flexor hallucis longus and flexor digitorum longus.
The two-incision technique is recommended by the British Orthopaedic Association and British Association of Plastic Reconstructive and Aesthetic Surgeons [41] (Fig. 4). The anterolateral incision is placed halfway between the tibial crest and the shaft of the fibula over the anterior intermuscular septum. A long (20-25 cm) incision is made along the length of the leg. A transverse incision in the fascia then allows identification of the anterior intermuscular septum. The anterior and lateral compartments can then be released taking care to avoid the superficial peroneal nerve just posterior to the intermuscular septum. The medial incision is made about 2 cm behind the medial tibial border, ensuring a sufficient skin bridge (>5 cm) between the two incisions. Blunt dissection allows visualisation of the fascia. A transverse incision can then be made between the deep and superficial compartments. Both compartments can then be fully decompressed. The soleus is firmly adherent to the posterior tibia and may need to be released to adequately decompress the deep compartment.
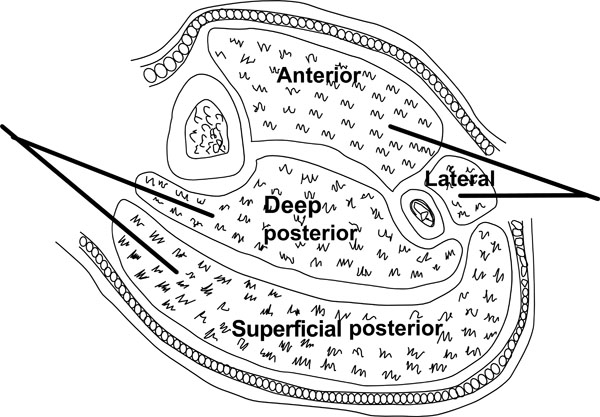
Fasciotomy of the leg.
THIGH
The thigh has three compartments (Fig. 5):
- Anterior compartment consisting of the quadriceps muscles
- Posterior compartment consisting of the hamstring muscles
- Medial compartment consisting of the adductor muscles
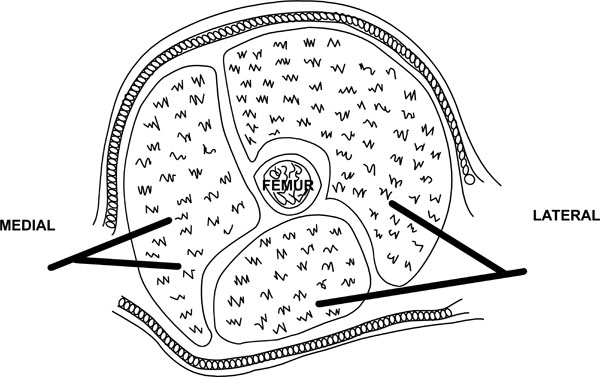
Approaches for thigh fasciotomy.
A single lateral incision is usually adequate to decompress the thigh as the medial compartment is only rarely involved. A long lateral incision is used spanning the length of the thigh. The fascia lata is incised in line of its fibres and the anterior compartment decompressed. The vastus lateralis can be retracted anteriorly to allow access to the posterior compartment for further decompression. As mentioned it is rare to need to decompress the medial compartment but if necessary a separate medial incision can be used (Fig. 5).
FOREARM
The forearm can be anatomically divided into three compartments (Fig. 6):
- Mobile wad comprising brachioradialis, extensor carpi radialis longus and brevis muscles
- Volar compartment comprising superficial and deep flexors
- Dorsal compartment containing the extensor muscles
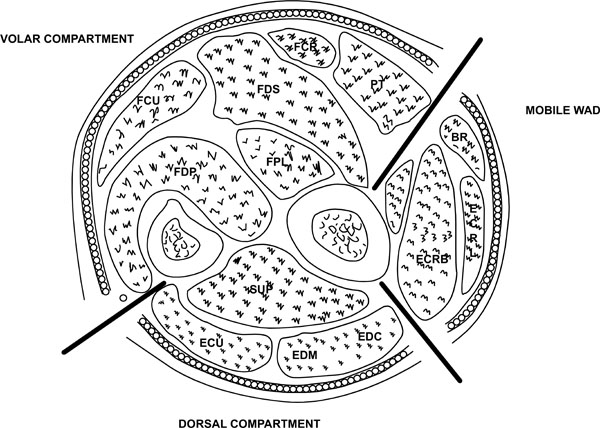
Compartments of the forearm.
Pronator quadratus may be described as a separate compartment.
A volar Henry approach provides exposure to adequately decompress the flexor compartments and the mobile wad. The incision is started 1 cm proximal and 2 cm lateral to the medial epicondyle extending to the mobile wad. It is carried distally to the midline down to the proximal wrist crease. Median nerve decompression is always performed at the same time. To decompress the dorsal compartment a single straight incision from the distal aspect of the lateral epicondyle aiming to the centre of the wrist (Thompson’s approach) can be utilised.
HAND
The hand has ten separate osseo-fascial compartments:
- Four dorsal interossei
- Three palmar interossei
- The thenar and hypothenar compartments
- Adductor pollicis
Fasciotomy is achieved using a four-incision technique (Fig. 7). One is on the radial side of the thumb releasing the thenar compartment. Two dorsal incisions are made over the index metacarpal and over the ring metacarpal. These incisions are used to release the dorsal and volar interossei. Adductor pollicis can also be reached using the index metacarpal incision. The hypothenar muscles are released using an incision at the ulna aspect of the little finger.
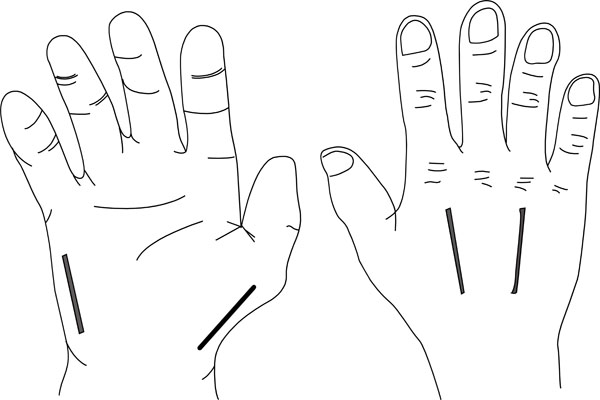
The four incisions to decompress the hand.
FOOT
Foot compartment syndrome is controversial both in terms of anatomy and in terms of treatment. Some authors advocate four compartments (medial, lateral, central and interosseous); others have expanded on this creating nine:
- Medial
- abductor hallucis
- flexor hallucis brevis
- Lateral
- abductor digiti minimi
- flexor digiti minimi brevis
- Interosseous (x4)
- Central (x3)
- superficial
- flexor digitorum brevis
- central
- quadratus plantae
- deep
- adductor hallucis
- posterior tibial neurovascular bundle
- superficial
Traditional treatment involves urgent fasciotomy using the following incisions (Fig. 8):
- 2 dorsal incisions overlying the 2nd and 4th metatarsals. Care is needed to maintain an adequate skin bridge. The superficial fascia is divided and the interossei are elevated off the metatarsals. Blunt dissection can then be continued through the central, medial and lateral compartments.
- A medial incision along the inferior border of the 1st metatarsal or a calcaneal incision beginning posteromedially towards the 1st metatarsal will allow more extensive decompression of the central compartment, which may be helpful with hindfoot involvement.
More recently, however, some authors now suggest a ‘supervised neglect’ approach accepting the inevitable deformity and clawing and suggesting reconstruction at a later date to avoid the high risk of potential surgical complications.
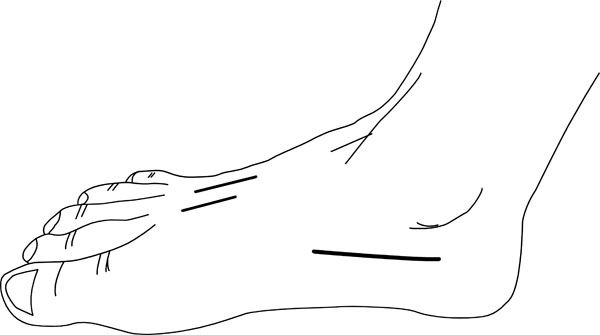
Incisions for foot fasciotomy.
DELAYED FASCIOTOMY
Fasciotomy after 8 hours in cases of ACS is controversial. The myoneural damage is irreversible at this stage and the increased risks may outweigh any potential benefit. Finkeltstein et al. described five patients with an average of 56 hours delay from the diagnosis in whom a fasciotomy was performed. One died from multi-organ failure, the remaining four required a later amputation [42]. Williams et al. found a rate of infection of 28% when fasciotomy was delayed more than twelve hours.
Where the diagnosis has been missed or delayed supportive renal treatment should be considered and surgery delayed until the morbidity has been declared and reconstruction can be planned. The evidence for this has generally come from traumatic cases. In certain cohorts (particularly haemophiliacs) this may not necessarily be the case and anecdotal evidence suggests better outcomes with delayed fasciotomy as clotting deficiencies are addressed prior to surgery.
WOUND MANAGEMENT AND WOUND COMPLICATIONS
Management of fasciotomy wounds remains controversial. Most advocate leaving the wounds open with delayed primary closure or skin grafting within 7-10 days when the compartment syndrome has completely resolved. A second look and debridement is usually necessary at 48-72 hours. Interim coverage can be achieved with simple absorbent dressings, semi-permeable membranes, vessel loops in a ‘bootlace’ pattern or with negative pressure dressings, which can be helpful in allowing later closure [44].
Fasciotomies are not benign procedures and there are multiple associated complications [45]:
- Altered sensation within the margins of the wound (77%)
- Dry, scaly skin (40%)
- Pruritus (33%)
- Discolored wounds (30%)
- Swollen limbs (25%)
- Tethered scars (26%)
- Recurrent ulceration (13%)
- Muscle herniation (13%)
- Pain related to the wound (10%)
- Tethered tendons (7%)
- Chronic venous insufficiency due to impaired calf muscle pumps [46].
COMPARTMENT SYNDROME IN HAEMOPHILIA
The lack of a clear aetiology or conspicuous traumatic injury both contribute to a potential diagnostic difficulty in haemophiliacs. In many cases the diagnosis of a bleeding disorder may not be known. The role of fasciotomy in haemophilia has been downplayed and care focuses on haemostatic manoeuvres in the first instance [47]. The absent clotting factor should be substituted and specialist haematological input sought. If these manoeuvres fail, fasciotomy is often performed to prevent muscle necrosis and future joint contracture. Caution should, however, be advised as surgical decompression can be catastrophic if the bleeding cannot be controlled and there is a higher than average amputation rate in haemophiliacs [48, 49].
MEDICO-LEGAL PITFALLS
ACS is a common cause of litigation. In nearly all cases, compartment pressures are never measured. Other potential pitfalls include malpositioning of the compartment pressure monitor, equipment errors and failure to correlate pressure reading with the clinical findings.
CONCLUSION
ACS is a surgical emergency and a high level of suspicion is needed in all potential cases. Compartment pressure monitoring may aid in the diagnosis, with a delta pressure of 30 mmHg or below suggestive of ACS. The definitive treatment is prompt surgical decompression of all the involved compartments. A delay of more than six hours is associated with irreversible myoneural damage and timing is crucial. Delayed fasciotomy after 8-10 hours is associated with significantly increased risks which may outweigh any potential benefit.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


