RESEARCH ARTICLE
Hyaluronic Acid (HA) Viscosupplementation on Synovial Fluid Inflammation in Knee Osteoarthritis: A Pilot Study
Heather K Vincent*, 1, Susan S Percival2, Bryan P Conrad1, Amanda N Seay1, Cindy Montero1 , Kevin R Vincent1
Article Information
Identifiers and Pagination:
Year: 2013Volume: 7
First Page: 378
Last Page: 384
Publisher ID: TOORTHJ-7-378
DOI: 10.2174/1874325001307010378
Article History:
Received Date: 14/6/2013Revision Received Date: 27/6/2013
Acceptance Date: 24/8/2013
Electronic publication date: 20/9/2013
Collection year: 2013

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.5/) which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective:
This study examined the changes in synovial fluid levels of cytokines, oxidative stress and viscosity six months after intraarticular hyaluronic acid (HA) treatment in adults and elderly adults with knee osteoarthritis (OA).
Design:
This was a prospective, repeated-measures study design in which patients with knee OA were administered 1% sodium hyaluronate. Patients (N=28) were stratified by age (adults, 50-64 years and elderly adults, ≥65 years). Ambulatory knee pain values and self-reported physical activity were collected at baseline and month six.
Materials and Methods:
Knee synovial fluid aspirates were collected at baseline and at six months. Fluid samples were analyzed for pro-inflammatory cytokines (interleukins 1β, 6,8,12, tumor necrosis factor-α, monocyte chemotactic protein), anti-inflammatory cytokines (interleukins 4, 10 13), oxidative stress (4-hydroxynonenal) and viscosity at two different physiological shear speeds 2.5Hz and 5Hz.
Results:
HA improved ambulatory knee pain in adults and elderly groups by month six, but adults reported less knee pain-related interference with participation in exercise than elderly adults. A greater reduction in TNF-α occurred in adults compared to elderly adults (-95.8% ± 7.1% vs 19.2% ± 83.8%, respectively; p=.044). Fluid tended to improve at both shear speeds in adults compared to the elderly adults. The reduction in pain severity correlated with the change in IL-1β levels by month six (r= -.566; p=.044).
Conclusion:
Reduction of knee pain might be due to improvements in synovial fluid viscosity and inflammation. Cartilage preservation may be dependent on how cytokine, oxidative stress profiles and viscosity change over time.
INTRODUCTION
Knee osteoarthritis (OA) may be triggered by excessive joint forces, defects to the articular cartilage or subchondral bone. Chondrocytes become metabolically active and initiate inflammatory processes that degrade articular cartilage and subchondral bone. Chondrocytes secrete several inflammatory cytokines that work synergistically to stimulate synthesis of enzymes that break down cartilage. Key cytokines include interleukin-1 (IL-1β), tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) [1]. IL-1β and TNF-α are commonly increased in inflamed joints, and these cytokines activate other inflammatory chemokines such as monocyte chemotactic proteins and others [2]. Hence, inflammation is a critical modifier of joint disease and progression.
Intriguing, but very limited evidence also suggests a role for oxidative stress-related degradation processes in human OA [3]. Oxidative stress represents the imbalance between endogenous antioxidant defenses and the free radical induced pro-oxidant processes that result in oxidative modification of molecules. Relevant to osteoarthritis, byproducts of lipid oxidation such as 4-hydroxynonenal (4-HNE) induce cell damage and death of chondrocytes [4, 5]. An imbalance of antioxidant defenses relative to oxidative processes has been shown to exist in human OA [3, 6]. The levels of oxidatively damaged byproducts such as lipid peroxides, are high in synovial fluid in patients with OA [3, 6]. These adverse changes correspond with cartilage breakdown.
Normally, synovial fluid contains high levels of hyaluronic acid (HA) that help to maintain high fluid viscosity and the normal integrity of the joint by attenuating inflammation and preserving the normal cartilaginous matrix. In OA, the synovial fluid viscosity and elasticity are decreased [7, 8]. HA is a polysaccharide produced by the chondrocytes and synoviocytes. While HA may help to lubricate and cushion the joint [9], it can help maintain cartilage matrix and minimize inflammation. In OA, the molecular weight and concentration of HA are reduced [10], thereby lowering fluid viscosity and elasticity. Protection against articular injury is compromised and OA damage ensues. In vitro data suggest supplemental HA can suppress IL-1β production [11], and may increase synovial fluid viscosity [10]. We hypothesize that intraarticular HA can suppress not only IL-1β, but also can reduce the overall inflammatory cytokine response in human OA. Clinical practice and anecdotal evidence suggest that HA may be more beneficial in mild to moderate OA [12]. However, most of evidence on disease severity and age has been derived from animal models of OA [13, 14]. Human studies have found that patients>60 years with higher disease severity responded better to HA than counterparts younger than 60 years [15]. Identification of the patient type with better responsiveness to HA would be an important next step in optimizing OA treatment for this clinical population.
Although published data on this topic are limited, we surmise that HA may be important in suppressing oxidative stress by reducing toxic oxidative byproducts [16] such as 4-HNE in the synovium. This suppression might be related to improvements in knee pain symptoms, improvements in physical activity and synovial fluid viscosity. These issues remain unclear at the present time. Therefore, the primary purpose of this study was to compare the six month changes in synovial fluid cytokine levels, 4-HNE and fluid viscosity after an intraarticular HA injection series in adults and elderly adults with knee OA. The secondary purpose was to determine whether there were improvements in knee pain and physical activity levels. This information will enhance our understanding of the mechanisms of joint repair and functional outcomes with intraarticular HA.
MATERIALS AND METHODOLOGY
Study Design
This was a prospective, repeated-measures study design in which the effects of a HA viscosupplement injection series on inflammatory parameters and viscosity of knee synovial fluid aspirates were examined. Pre-injection and month six levels of synovial fluid biomarker levels (inflammatory, oxidative stress) and fluid viscosity were measured. This study was approved by the University of Florida Institutional Review Board (UFIRB), and all procedures on human subjects were conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000. All patients read, understood and signed an informed consent document.
Patients
Patients (N=28) with chronic knee OA were recruited from the UF Orthopaedics Clinics. The inclusion criteria were a diagnosis of knee OA, degenerative joint disease or joint degeneration (these diagnoses were primary or secondary due to trauma or a sports injury). Patients had to be fully cognizant of all study procedures and willing to withhold taking any new knee OA medications for six months. The exclusion criteria were: allergic to hyaluranons, currently experiencing a knee infection or skin infection around the injection site, other current skin disease and wheelchair dependent. Patients were stratified into adults (50-64 years) and elderly adults (≥65 years). The age cutoffs were chosen to represent the age at which OA becomes one of the top co-morbidities in men (50 years) [17], and a general accepted definition of elderly (>65 years). A group of controls was not approved for this study due to the elevated risk to benefit ratio of obtaining intraarticular fluid samples in persons not receiving a treatment. Weight bearing anteroposterior knee radiographs were classified according to the Kellgren–Lawrence (KL) radiographic rating scale [18] by the study physician (KRV). All patients read, understood and signed a UF IRB approved informed consent form. The selection of 28 participants was based on the ability to detect a minimal clinically relevant reduction in knee pain severity of 30% by month six [19], and a ~50% decrease in IL-1β similar to that observed in HA injection in other articular joints [20].
Patient History and Physical Activity Patterns
All patients completed a specific health history questionnaire which included demographic data, current and past medical issues, current medications, knee OA history and current medications. The current physical activity patterns were captured by documenting the type of exercise, the number of weekly activity sessions, and the average session duration in block levels. The duration block levels were: 1) 10-15 min, 2) 15-30 min, 3) 30-45 min, 4) 45min-1 hr and 5) ≥1 hour. For example, a participant who walked three times a week for 45 minutes would have the exercise pattern entered into the statistical program as: walking (activity type), 3 (frequency per week), block 3 (duration of walking). Knee pain severity was self-assessed during walking activity using an 11 point numerical pain rating scale (NRSpain) with terminal anchors (0 = “no pain”; 10 = “worst possible pain”). The NRSpain is an accepted outcome measure for chronic pain conditions, as described in the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials [21]. This measurement is reliable and valid [22] for assessing pain intensity.
Intraarticular Injection Procedure for Hyaluronic Acid
The patient sat with the knee(s) flexed at 90º. The injection site was cleansed with chlorhexidine, and local anesthesia was provided to the pathway for the injection using three mL of 1% lidocaine. An anteromedial or lateral approach was performed for these injections. A 20g needle with a 20 ml syringe was utilized to withdraw synovial fluid aspirate to be used for subsequent analysis. The aspirate was immediately aliquoted and frozen at -70°C. A preloaded syringe of two ml of viscosupplement (Euflexxa®.) was then delivered through a 20-gauge 1½ inch needle using the anteromedial or lateral approach. The patients were observed for 15 minutes post-procedure. Two more injections were provided to each patient at weekly intervals for a total of three injections.
Synovial Aspirate Analyses
A series of cytokines and oxidative stress measurements and viscosity measures were collected from synovial fluid aspirates at baseline and month six.
Cytokine Assessments
A Milliplex MAP kit of Human Cytokines/ Chemokines (cat #s HCYTOMAG-60K, 60K-PX29, 30, 39, 42; Millipore Corp; Billerica, MA) was used for the simultaneous quantification of several cytokines. These included inflammatory interleukins 1β, 6, 8, 12, monocyte chemotactic protein-1 (MCP-1), granulocyte macrophage stimulating factor (GM-CSF), interferon gamma (IFNγ) [2]. Anti-inflammatory cytokines included interleukins 4, 10 and 13., fluid mixtures of color coded microspheres with two fluorescent dyes were created and coated with antibodies specific to the cytokines measured. The synovial fluid samples were mixed with the dyed microspheres, and the cytokines were captured. The mixture was exposed to Streptavidin PE conjugate and passed through a laser. The cytokine levels were quantified by the amount of fluorescence of the sample using a multiplex plate reader (Luminex, Austin TX). Samples were run in duplicate; if one sample was markedly different than the other, a third sample was analyzed.
Synovial Fluid 4-Hydroxynonenal (4-HNE) Content
4-HNE may be involved in the pathogenesis of osteoarthritis via cartilage breakdown [23]. 4-HNE content was determined using an enzyme linked immunosorbent assay (OxiSelect ELISA, Cell Biolabs, Inc; San Diego CA; cat STA-338-5). Samples were run in duplicate; if one sample was markedly different than the other, a third sample was analyzed.
Viscosity Assessment
To determine whether the viscous properties of the synovial fluid aspirates changed from baseline to month six, a viscometer was used (Brookfield cone plate viscometer; Middleboro, MA; cp-52 cone, 3° angle). A 0.5mL sample of synovial fluid was placed into the viscometer, and a torque meter was driven at discrete rotational speeds. The internal measuring system was comprised of a spring mechanism connected to a rotating cone that senses resistance to rotation caused by the synovial samples. The resistance to rotation of the cone is proportional to the shear stress in the fluid; the reading was converted to centipoise units (cP). A circulating water bath maintained all sample temperatures at 25° C. The fluid viscosity was assessed at two different shear speeds (2.5Hz and 5Hz) to simulate how fluid might behave during stresses experienced during normal physiological activities such as walking [24, 25].
Statistics
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS, v. 20). Descriptive measures and frequencies were compared at baseline between the two age groups. Repeated measures analysis of variance (ANOVA) were performed on the biochemical and viscosity variables, where age group was the between factor (adults, elderly adults) and the within group factor was time (baseline, month 6). Greenhouse-Geisser corrections were used. Change scores in the cytokine levels and 4-HNE were determined (change from baseline to month six) and compared between age brackets using independent samples t-tests. These same analyses were performed using the KL OA staging (stage II, II, IV) as between group factors to determine the effect of OA stage on the study outcomes. Non-parametric tests (Mann Whitney U tests) were used to determine whether comparisons were found to be significant, and simple main effects were analyzed using a one way AONVA. Significance was established at p≤0.05 for all analyses.
RESULTS
Patients
Participant characteristics are shown in Table 1. By month six, two patients, one from each age group, had opted for knee replacement surgery because the pain did not improve. The six month data represent the patients who completed the study. Among all patients, there was an average reduction in pain severity from 7.8 ± 1.5 points to 3.8 ± 3.5 points (an average pain reduction of 51.2%; p<0.0001). There were no adverse events to report.
Baseline Participant Characteristics. Values are Means ± SD, or % of the Group
| Adults (n=14) | Elderly Adults (n=14) | |
|---|---|---|
|
|
||
| Age (years) | 57.3 ± 5.1 | 66.6 ± 1.3 |
|
|
||
| Height (cm) | 168 ± 8 | 167 ± 9 |
|
|
||
| Weight (kg) | 86 ± 16 | 86 ± 21 |
|
|
||
| Body mass index (kg/m2) | 30.1 ± 4.8 | 30.6 ± 5.1 |
|
|
||
| Women (%) | 57.1 | 57.1 |
|
|
||
| Race | ||
|
|
||
| Caucasian (%) | 92.9 | 78.6 |
| African-American (%) | 7.1 | 14.3 |
| Hispanic (%) | 0.0 | 7.1 |
|
|
||
| Working Status | ||
|
|
||
| Working (%) | 57.1 | 42.9 |
| Retired (%) | 7.1 | 50.0 * |
| Disabled (%) | 35.7 | 7.1 * |
|
|
||
| Kellgren Lawrence OA Stage | ||
|
|
||
| 2 (#) | 9 | 4 |
| 3 (#) | 3 | 6 |
| 4 (#) | 2 | 4 |
| Mean | 2.5 ± 0.8 | 3.0 ±0.8 |
|
|
||
| Pain in both knees (%) | 21.4 | 42.9 * |
|
|
||
| Knee pain duration (years) | 6.5 ± 9.3 | 11.1 ± 13.3 |
|
|
||
| Knee pain grown worse over last 6 months (yes, %) | 21.4 | 35.7 |
|
|
||
| Medication Use for Pain | ||
|
|
||
| NSAIDs (%) | 50.0 | 21.4 |
| Narcotics (%) | 35.7 | 71.4 * |
| Antidepressants (%) | 57.1 | 78.6 |
* Different at p<0.05.
Knee Pain Symptoms
The changes in knee pain symptoms related to physical activity are shown in Table 2. There was a significant group by time interaction, such that fewer adults reported that pain prevented exercise compared to elderly patients at six months (p<0.05). There was no change in the number of weekly physical activity sessions. There was, however, a trend toward a significant group by time interaction for an increased session duration from baseline to month six (p=0.07) suggestive of increased physical activity tolerance in the adults.
Changes in Knee Symptoms and Relation to Physical Activity from Baseline to Month Six. Values are Means ± SD, or % of the Group
| Adults | Elderly Adults | |||
|---|---|---|---|---|
| Baseline | Month Six | Baseline | Month Six | |
| OA pain medication (#) | 1.8 ± 1.5 | 0.9 ± 0.8 | 1.1 ± 0.7 | 1.2 ± 0.8 |
| Pain, walking (points) ** | 8.0 ±1.3 | 3.0 ± 5.0 | 7.6 ± 1.6 | 5.0 ± 4.0 |
| Pain prevents exercise (yes, %)* 78.6 | 33.3 | 78.6 | 41.7 | |
| Physical Activity | ||||
| AX≥ 3X/week (%) | 50.0 | 66.6 | 42.8 | 46.2 |
| Session duration level^ | 1.3 ± 1.5 | 2.3 ± 1.4 | 1.4 ± 1.6 | 1.8 ± 1.5 |
Note: pain was assessed using a 0-10 Numerical pain rating scale. AX = aerobic exercise.
^ Session duration: 1= 10-15 min, 2: 15-30 min, 3: 30-45 min, 4: 45min-1 hr and 5: ≥1 hour.
* The interaction of group by time, p<0.05;
** significant by time, p<0.05.
There was a trend for greater knee pain severity improvement in the stages II and III OA compared with stage IV OA (p=0.06); pain decreased by an average of 54% and 87% in patients with stages II and III compared to a 19% improvement in patients with stage IV OA.
Cytokine and 4-HNE
The baseline and six month cytokine levels are shown in Table 3.
Cytokine Profiles from Baseline to Post-HA Viscosupplementation at Month Six. Values are Expressed in pg/ml and are Shown as Means ± SD
| Adults | Elderly Adults | |||
|---|---|---|---|---|
| Baseline | Month 6 | Baseline | Month 6 | |
| Inflammatory | ||||
| IL-1β | .068 ± .192 | .298 ±.595 | .333 ±.763 | .107 ±.223 |
| IL-6 | 429.5 ± 412.3 | 348.9 ± 372.8 | 187.7 ± 156.9 | 231.5 ± 257.4 |
| IL-8 | 92. 4 ± 73.0 | 67.1 ± 23.0 | 117.1 ± 106.7 | 162.8 ± 155.1 |
| IL-12 | 2.29 ± 1.96 | 1.64 ± 1.68 | 0.81 ± 1.36 | 1.59 ± 1.95 |
| TNF-α* | 2.52 ± 3.09 | 1.33 ± 2.75 | 3.76 ± 3.71 | 5.38 ± 4.54 |
| MCP | 2797 ± 990 | 2189 ± 786 | 3117 ± 1397 | 3611 ± 1192 |
| Anti-Inflammatory | ||||
| IL-4 | 2.8 ± 3.3 | 5.7 ± 8.6 | 10.2 ± 13.9 | 5.8 ± 6.7 |
| IL-10 | 8.42 ± 6.47 | 6.39 ± 3.03 | 8.37 ± 5.04 | 8.40 ± 7.67 |
| IL-13 | .565 ± .780 | 0.299 ± .745 | .606 ± .780 | 0.081± .229 |
Intxn= group by time interaction.
IL= interleukin; TNF= tumor necrosis factor; MCP = monocyte chemotactic protein.
* Change scores (difference between baseline and six months) between groups were different p0.<05.
While there were general reductions in the inflammatory cytokine levels in the adults compared to the elderly adutls, these were not significant. There was a significant reduction in TNF-α from baseline to month six in adults compared with the elderly adults (p=0.044). Correlations revealed that the reduction in pain severity was moderately related with the change in IL-1β levels by month six (r= -.566; p=0.044). The changes in 4-HNE are shown in Fig. (1). While the increase in 4-HNE was less in adults compared to the elderly adults by month six (7% vs 21%), this difference was not found to be significant. Analyses revealed that none of the biochemical variables were different based on KL OA stage from baseline to month six (all p>0.05).
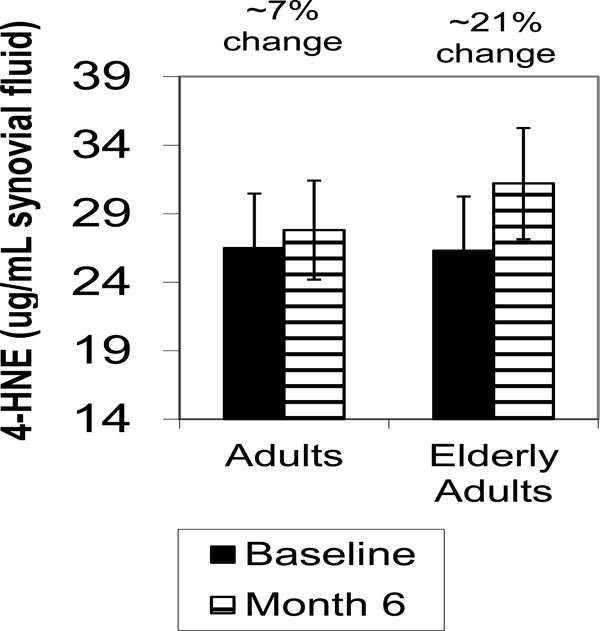 |
Fig. (1). 4-Hydroxynonenal (4-HNE) levels in knee synovial fluid before and six months after intra-articular HA injections in adults and elderly adults. Values are means ± SD. |
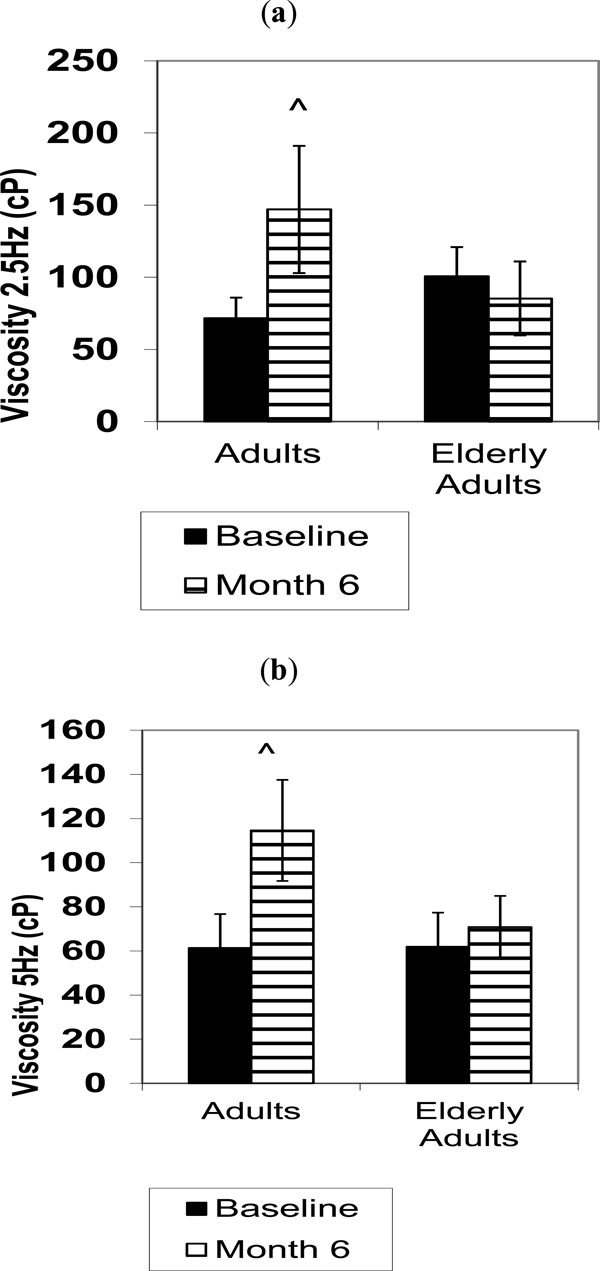
SYNOVIAL FLUID VISCOSITY
The change in viscosity between groups from baseline to month six tended to be greater in at the 2.5 Hz and 5Hz shear speed conditions in the adults compared to the elderly adults (Fig. 2a [p=0.07] and Fig. 2b [p=0.08]). Analyses revealed that the viscosity measures were not different based on KL OA stage from baseline to month six (all p>0.05).
DISCUSSION
The main purpose of this pilot study was to compare the changes in synovial fluid cytokine levels, 4-HNE and fluid viscosity by six months after an intraarticular HA injection series in adults and elderly adults with knee OA. This was a first step in examining the HA mechanisms of pain change and OA processes in these two age groups. The main findings included a significant reduction in knee pain with HA by six months, and this was more improvement in pain severity than previously reported [26]. Pain reductions were accompanied by age-dependent improvements in fluid viscosity at higher shear speeds and a greater reduction in TNF-α in the elderly adults. Adults aged 50-64 reported less knee pain-related interference with participation in exercise than the elderly adults 65 years and older. While we did not find significant differences in pain relief among patients with different OA severity stages, we found a trend. The effects of HA likely involve a collective favorable improvement in pro- and anti-inflammatory cytokines, viscosity and oxidative damage in the synovial fluid.
Available comparative biochemical data have been derived largely from cell culture or animal studies. Fibroblast like synoviocytes isolated from synovial fluid samples from patients with early stage OA (N=15) were exposed to high molecular weight HA in vitro [27]. HA down-regulated TNFα and IL-8 after 24 hours, indicating an anti-inflammatory effect. In degraded bovine cartilage tissue models in vitro, 1% HA solution promoted proteoglycan synthesis. The authors proposed that the superficial damaged synoviocytes permitted penetration of HA into the cartilage matrix for repair [28]. Rat models revealed that IL1β induced chondrocyte apoptosis in vitro and found dose-dependent protection of HA against cell death [29]. In the rabbit knee OA model, HA treatment suppressed metalloproteinase and prostaglandin production over the short term, leading to better weight tolerance of the affected limb [30]. In patients with OA, HA has been shown to reduce synovial fluid IL-6 levels more than saline at one week (47% vs 33% reduction), but did not decrease TNF-α levels [31] as we observed in the present study. Two favorable trends in IL-4 elevations and monocyte chemotactic protein decreases occurred in the adults compared to the elderly. The different responses among studies may be due to the variations of HA preparations, the wide range of measurement time points after treatment, and the initial level of physical function.
Synovial fluid viscosity tended to increase by six months after HA treatment in both shear stress conditions in the adults compared to the elderly adults. Improvements in viscosity have been documented in OA (16%) [10], in persons with stage III knee gonarthrosis [32] and in rheumatoid arthritis [33] by three to six months post-injection. Our study expanded the viscosity measures to two different shear speeds to simulate different knee loading conditions. The significant increase in viscosity at higher shear speeds the adults concurrent with pain reduction suggests that either the quality of the synovial fluid by month six has improved or viscosity alters nocioceptive nerve activity, or both. Animal models of knee inflammation indicate that intraarticular injections of elastoviscous HA can reduce movement-induced nocioceptor impulse discharges [34] and reduce pain. There is the possibility that patients experience different HA effects depending on age or disease severity. For example, despite increases in inflammatory cytokines and 4-HNE and no change in viscosity, the elderly group reported lower knee pain severity. Perhaps this group experienced nocioceptive change. These data indicate that adults had better quality synovial fluid by month six coupled with lower cytokine levels, and pain relief may have been mediated by different chemical pathways. These temporal patterns of pain change relative to physiological pathways in the knee joint need additional investigation.
STUDY LIMITATIONS AND FUTURE DIRECTIONS
Limitations to this study deserve comment. The lack of a formal control group was a limitation, but this was due to the higher risk to benefit ratio for persons who would not receive treatment. The study design used here has been used in a previous hyaluranon study in humans, in which sequential samples over time were collected to determine synovial fluid changes after intraarticular injection [35]. We believe that this study design provides valuable information regarding the individual responses to treatment. The relatively small sample size was related with variability in some of the synovial fluid biomarkers. We found intriguing trends in graded improvements in synovial fluid viscosity and knee pain symptoms based on age and OA stage. Larger studies are needed to confirm these findings. Previous cell culture work using human chondrocytes from patients with OA show variability in responsiveness to cytokines [35], indicating that there may be better responders to treatment. The fact that there was a greater percentage of persons with knee pain in both knees in the elderly adults than in the adults could influence biochemical responsiveness to the HA and might be a confounder. That is, a long exposure to OA over years could attenuate responsiveness of the cytokine pathways to treatment. The elderly adults had similar reductions in pain severity despite less change in cytokine profiles than the adults, so this could mean that pain changes are less dependent on inflammatory cytokine levels with aging.
Because there were patterns of improvement in the adults (Table 3; less increase in 4-HNE, lower IL-6, IL-8, IL-12 and MCP values, higher IL-4 levels) suggests that there may be benefit to administering HA after less exposure time to OA. Adults may be more responsive to the HA than elderly adults. Comparative evidence on this topic is scarce, but animal model data support the notion that early treatment with HA can limit future degenerative changes within the knee joint [36]. Additional studies are needed to determine whether there is an age-related threshold at which the HA improvements in pain and inflammation are minimal. We did not anticipate being unable to obtain synovial fluid aspirates in some patients by month six. This challenge has been reported in other HA studies [10, 33] and could signify that the components of the synovial fluid were being laid down as matrix in the damaged cartilage, and this warrants further investigation. Relationships between synovial fluid inflammation, oxidative stress, cartilage volume preservation and physical function should be examined across the age span to determine which patient populations may obtain the best benefit from HA treatment.
CONCLUSION
Compared to the elderly adults, adults reported less knee pain-related interference with participation in exercise by six months after HA treatment. Both age groups experienced less ambulatory pain by month six. Mitigation of pain might be due to an improvement in synovial fluid viscosity, reduction in inflammation. Longer prospective studies are needed to determine whether or not there is differential cartilage preservation based on age and OA stage and how cytokine, oxidative stress profiles and viscosity change over time.
CONFLICT OF INTEREST
This project was supported by an Investigator Initiated grant from Ferring Pharmaceuticals, 2010-2012 (HK Vincent, A Seay). The study sponsor had no involvement in any phase of the study conception, execution and manuscript preparation.
ACKNOWLEDGMENTS
The authors sincerely thank the contributions in data collection from Ms. Kelley Lamb, the equipment loan from Dr. Roger Son-Tay and the technical expertise of Ms. Joy Stanilka. The investigators also thank the study patients for their willingness to assist in this project.