All published articles of this journal are available on ScienceDirect.
Economical Analysis on Prophylaxis, Diagnosis, and Treatment of Periprosthetic Infections
Abstract
The economic burden of periprosthetic infections is enormous, but the number of economic studies dealing with this issue is very scarce. This review tries to know the economic literature existing, assess the value of current data, and recognize the less costly and more effective procedures for prevention, diagnosis and treatment of periprosthetic infections.
Forty five studies meeting the inclusion criteria and adhering to the quality criteria used were carefully analyzed to extract the economic data of relevance in evaluating the magnitude of problem and the more cost-effective solutions. However, because the heterogeneity and the low-quality of most of these studies meta-analytical technique has not been possible. Instead, the studies have been reviewed descriptively.
Optimizing the antibiotic use in the prevention and treatment of periprosthetic infection, combined with systemic and behavioral changes in the operating room; detecting and treating the high-risk groups; a quick, simple, reliable, safe, and cost-effective diagnosis, and the rationale management of the instituted infection, specifically using the different procedures according to each particular case, could allow to improve outcomes and produce the highest quality of life for patients and the lowest economic impact. Nevertheless, the cost effectiveness of different interventions to prevent and to treat the periprosthetic infection remains unclear.
INTRODUCTION
Many publications addressing periprosthetic infection (PPI) have remarked the condition of “devastating” complication, with a risk around 1% after total hip arthroplasty (THA) [1-6], and between 1 and 2% after total knee arthroplasty (TKA) [2, 3, 7-9]. Infection was the cause of 14.8% of revisions of THA [10], and the most common cause of revision TKA in 25.2% [11]. Rehospitalization, at least one time, for deep infection in the first year after primary THA or TKA, occurs in 1.3% of patients, being revised 26% of them [12]. The economic burden of PPI is expected to exceed 50% of the inpatient resources spent in revisions by 2016 for TKA and by 2025 for THA [13]. All this supposes a substantial economic burden on patients, physicians, hospitals, health-care systems and society in general.
The optimization of existing resources compels the healthcare professionals to analyze in depth and critically the value of different preventive, diagnostic and therapeutic methods and technologies, to provide cost-efficient high-quality cares. As just has been said, is very important correlating outcomes with expenses incurred to achieve them. The identification and valuing of costs may be an added step of decision analysis able to evaluate multiple competing strategies, considering differential risks, costs and benefits, with various potential outcomes, aiding to find the most profitable option, especially when substantial uncertainty exists or when the timing of subsequent events is important. Economic and decision analyses are evidence-based tools to take correct choices. The guidelines of these economic analyses have been reported in the literature and must be understood and used to compare procedures and to choose the best option [14-24].
While the favorable cost effectiveness of primary or revision THA and TKA has been demonstrated [25-30], there is not the same certainty regarding the management of PPI [31, 32]. Determining the less expensive prophylactic, diagnostic and therapeutic methods that best control infection and at the same time improve outcome and minimize patient morbidity and mortality, might produce the highest quality of life for patients and the lowest economic impact on the healthcare systems as well the society in general. This review tries to know the economic literature existing, assess the value of current data and recognize the less costly and more effective procedures for prevention, diagnosis and treatment of PPI.
MATERIALS AND METHODOLOGY
In May 2012, a systematic review of the literature was performed using a computerized search of MEDLINE/PubMed, Embase, NHS EED, CEA Registry, and Cochrane databases. “Periprosthetic infection”, “infected total hip arthroplasty”, “infected total knee arthroplasty”, “diagnosis”, “screening”, “prevention”, “prophylaxis”, “treatment”, “revision”, “economic”, “cost”, “cost effectiveness”, “cost utility” and “decision analysis” were the concepts used to search the interesting articles related to the focus of the review. Search strategy developed by the first author and a research librarian is listed in Table 1. All types of study designs were accepted for inclusion in this review. Languages included were English, Spanish, French, German and Italian.
Search Performed in the Following Numerical Order (Pubmed/Embase)
| #1 | Total joint arthroplasty OR Total joint replacement OR Total joint prosthesis |
| #2 | Total hip arthroplasty OR Total hip replacement OR Total hip prosthesis |
| #3 | Total knee arthroplasty OR Total knee replacement OR Total knee prosthesis |
| #4 | Total joint arthroplasty infection OR Total joint replacement infection OR Total joint prosthesis infection |
| #5 | Total hip arthroplasty infection OR Total hip replacement infection OR Total hip prosthesis infection |
| #6 | Total knee arthroplasty infection OR Total knee replacement infection OR Total knee prosthesis infection |
| #7 | Periprosthetic infections |
| #8 | Infection prophylaxis OR infection prevention |
| #9 | Infection diagnosis OR infection screening |
| #10 | Infection treatment |
| #11 | Antibiotics |
| #12 | Debridement-retention |
| #13 | One stage revision OR 1stage revision |
| #14 | Two stage revision OR 2 stage revision |
| #15 | Delayed re-implantation OR stage re-implantation OR staged revision |
| #16 | Resection arthroplasty |
| #17 | Arthrodesis |
| #18 | Amputation |
| #19 | Financial burden |
| #20 | Economics |
| #21 | Cost OR cost analysis |
| #22 | Cost effectiveness |
| #23 | Cost utility |
| #24 | Cost benefit |
| #25 | Decision analysis |
| #26 #1 OR #2 OR #3 | |
| #27 #4 #5 OR #6 OR #7 | |
| #28 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 | |
| #29 #8 OR #9 OR #28 | |
| #30 #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 | |
| #31 #26 AND #27 AND #29 AND #30 | |
The initial search recovered 557 references (Fig. 1). Whenever possible, relevance based on the abstract was judged and in cases where no abstract was available, the full-length text was obtained. Reference lists of acquired full-length publications were also examined by a hand search and performing a citation search of key articles in the ISI Web of Science portal. Two of the authors (MF-F and AT) reviewed the search results independently and decided whether the articles should be included, should be excluded, or whether this was unclear.
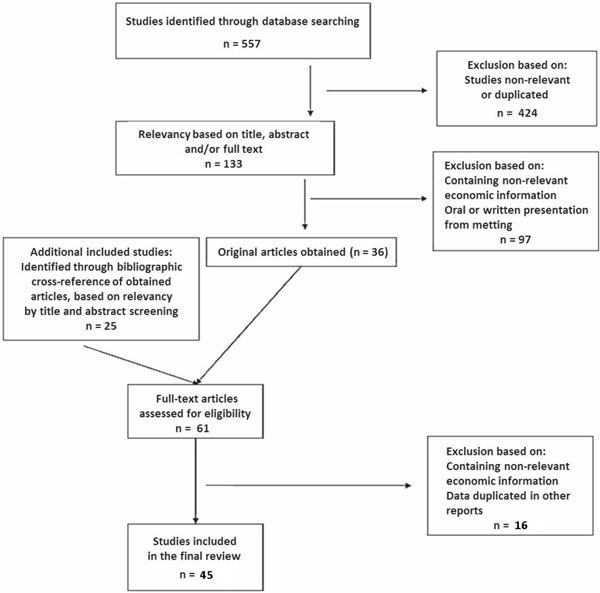
Flow diagram through the different phases of the review.
The two reviewers’ lists of papers that should be included were compared to each other, and where there was any discrepancy, they were re-classified according to the consensus reached. Studies with overlapping data were assessed and the most appropriate one was chosen for inclusion. Studies identified as oral or written presentation from meeting were not included.
The studies were incorporated by meeting the following inclusion criteria: i) formal economic evaluation, ii) an intervention specific to hip or knee periprosthetic infection and iii) evident perspective of the study. Each publication that met the established guidelines for one of the four basic types of economic analyses, cost-identification, cost-effectiveness, cost-utility, and cost-benefit [33-35] was then critically reviewed for content and method used. Data collected and examined from each study were the subject of the investigation, the perspective taken, the source and type of cost data used, the time-horizon considered, the cost-effectiveness ratios or other economic measures, and the discounting and sensitivity analysis employed. The methodological consistency of studies was assessed on basis of their adherence to the principles announced by Udvarhelyi et al. [23] (Table 2), and Drummond et al. [36], as common standards required for healthcare economic analysis. They were subsequently stratified based on the following criteria:
Principles Recommended by Udvarhelyi et al. [23] to Report Healthcare Economic Analysis
|
- What was the function of the intervention assessed (ie, preventive, diagnostic, or therapeutic)?
- What was the nature of the intervention (ie, drug, type of equipment, surgical procedure, or healthcare system)?
- What was the type of economic evaluation (ie, cost minimization, cost benefit, cost-effectiveness, or cost utility)?
- What was the level of scientific evidence (SE) of the study?
The articles selected following this strategy were categorized in three groups according to the subtheme treated: prevention, diagnostic or therapeutic studies; in four subgroups depending on the type of study performed: cost-identification, cost-effectiveness, cost-utility, and cost-benefit analysis, as defined by Robinson [34] (Table 3); and according to level of SE [37]. Any publication containing cost data, but not including any measurement of or correlation with outcome measures was considered as a cost-identification study.
The Four Mean Approaches in Use for Healthcare Economic Evaluation [34]
|
Development and reporting of this study were done following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guideline [38-40]. The heterogeneity of studies, the lack of details in reporting materials and methods, and the huge methodological deficiencies observed, made impossible to perform a meta-analysis. Instead, a descriptive review of results and conclusions of retrieved papers is presented.
Figures reflected in text and in Table 4 are those originally expressed in the different studies. The diverse currencies expressed in these studies have been converted in US$ according to the appropriate historical exchange rates. To compare costs in other tables, they were updated using rates of inflation from the date of publication until the present time. When current prices of products being well known as those of the generic antibiotics have been compared with the old prices. The 2012 List of Drug Prices of the Colegio Oficial de Farmaceúticos de Madrid [41] and the Medscape Reference Drugs, Diseases & Procedures [42] were used to know current prices of drugs.
Average Cost in 1996/1997 US$ of PPI Prophylaxis Using Parenterally and Locally Administered Antibiotics, Surgical Enclosure and Ventilated Suits, Alone or in Combination (Data from Persson et al. [77])
| Method of Prophylaxis | N° of TJA Per Year | ||||
|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | |
| Systemic antibiotics | 33 | 33 | 33 | 33 | 33 |
| Gentamicin impregnated bone cement | 134 | 134 | 134 | 134 | 134 |
| Exhaust ventilated suits | 94 | 72 | 65 | 61 | 59 |
| Surgical enclosure | 329 | 164 | 110 | 82 | 66 |
| Systemic antibiotics + gentamicin impregnated bone cement | 166 | 166 | 166 | 166 | 166 |
| Systemic antibiotics + exhaust ventilated suits | 126 | 105 | 97 | 94 | 92 |
| Systemic antibiotics + surgical enclosure | 361 | 197 | 142 | 115 | 98 |
| Gentamicin impregnated bone cement + surgical enclosure | 463 | 298 | 243 | 216 | 200 |
| Exhaust ventilated suits + surgical enclosure | 423 | 236 | 175 | 143 | 125 |
| Systemic antibiotics + gentamicin impregnated bone cement + surgical enclosure | 495 | 331 | 276 | 249 | 232 |
| Systemic antibiotics + surgical enclosure + exhaust ventilated suits | 455 | 269 | 207 | 176 | 157 |
| Gentamicin impregnated bone cement + surgical enclosure + exhaust ventilated suits | 556 | 370 | 308 | 277 | 263 |
| Systemic antibiotics + gentamicin impregnated bone cement + surgical enclosure + exhaust suits | 589 | 403 | 341 | 310 | 291 |
RESULTS
Forty original studies published in English [43-82] and 5 studies published in other languages [83-87] between 1984 and 2012 were included (Fig. 1). Six were published between 1984 and 1990, 8 between 1991 and 2000, and 31 between 2001 and 2012. Of the 45 included studies, 30 (67%) were identified by the electronic-database search.
A large segment of these publications was studies evaluating clinically different procedures, with some economic data inserted into them. Most have been considered simple cost-identification analyses. Only 12 studies (26.6%) adhered to established criteria for a comprehensive health care economic analysis.
Seventeen (38%) dealt with prophylaxis, 3 (7%) with diagnosis, and 25(55%) with treatment of PPI. Thirty two (71%) were classified as cost-identification analyses, 12 (27%) as cost-effectiveness analyses, and 1 (2%) as cost-utility analyses. There was no cost-benefit study. None of these studies had a level I of SE, five (11%) had a level II of SE, and twelve (27%) had a level III.
Results of Economic Studies on Prevention of Periprosthetic Infection
The less expensive tactic against the periprosthetic infection remains prevention. The attainment of effective, low-cost, safe, and easy to use methods to elude periprosthetic infection is certainly the most logical approach. In that sense, sixteen articles dealing with prophylactic measures to avoid arthroplasty infection have been included in this section.
Five studies considered systemically administered antimicrobial prophylaxis in total joint replacement (TJR) [48, 53, 59, 61, 69]. Four were simple cost-identification analysis [48, 59, 61, 69], and one was a cost-utility analysis [53].
The efficacy and the cost-effectiveness of antibiotics to prevent PPI depend on the selected antibiotic, the required quantity per dose and number of doses. With a similar efficacy, safety, and prices, a prophylactic regimen with cefazolin (1987 US$6.55/g), cephalosporin of first generation, giving one preoperative dose of 1 g followed by 500 mg every eight hours for six doses, was cost savings in comparison with cefamandole (1987 US$6.99/g), cephalosporin of second generation, 2 g preoperatively and then 1 g every eight hours for six doses (1987 US$26.20 vs US$55.92) [48]. Cefamandole is no longer available in the United States or in Spain.
The efficacy of single dose or short-term prophylaxis regimen has been estimated as equivalent to that of long-term regimen, but reducing risk of adverse effects and bacterial resistance, and lower cost [56]. In 1986, when cefazolin was given in 1-g parenteral dose intraoperatively only or repeated every six hours for 24 hours, 48 hours, or seven days, the cost savings of intraoperative antibiotic regimen rather than for 48 hours were estimated as US$77 per case, and from seven days to one-dose antibiotic, the savings were estimated US$297 per patient without any difference in the infection rate [59]. The cost savings with current prices of these antibiotics could be US$31.45 per case of one dose versus 48 hours regimen and US$110.04 per case using one dose instead the seven days regimen.
From an RCT published in 1987, the single-dose of cefotaxime, cephalosporin of third generation, cost US$12.90; the previously used multi-dose of cefazolin cost US$30, and the five doses of cefoxitin, cephalosporin of second generation, cost US$100. The authors proposed a single 1-g dose of cefotaxime as a cost-effective prophylaxis alternative [61]. The current cost of these options, US$11.19 for cefotaxime, US$35.38 for cefazolin, and US$70.15 for cefoxitin, makes sustainable the mentioned statement to date.
Comparing in an RCT performed in 1994, cefuroxime, cephalosporin of third generation, in one preoperative dose of 1.5 g followed by 750 mg eight and sixteen hours later, for a total one-day antibiotic regimen, with cefazolin, 1 g every eight hours for nine doses, for a total of three days of antibiotic regimen, the infection rate in TJR was 0.5% for cefuroxime and 1.3% for cefazolin [69]. The total cost of prophylaxis per patient was calculated as US$37.03 for cefuroxime and US$56.07 for cefazolin. This difference is maintained in 2012(US$15.72 for cefuroxime and US$35.38 for cefazolin).
There is no evidence to suggest that new-generation cephalosporins or administration of antibiotic beyond 24 hours postoperatively is more effective at preventing postoperative PPI in THA/TKA surgery than first-generation cephalosporins or single-dose, or short-term administration. The use of one-dose first-generation cephalosporin is effective enough, reducing costs, risk of toxicity and the development of bacterial resistance [56, 88].
Regarding pejorative microorganisms such as methicillin-resistant Staphylococcus aureus (MRSA), a cost-utility study, with a level II of SE, has shown that prophylaxis using vancomycin, a glycopeptide, associated to cephalosporins is cost-effective for the prevention of MRSA infections after THA surgery when, with cephalosporin prophylaxis only, the rate of MRSA infection was 0.25% or more and the rate of other infections was 0.2% or more [53]. It must be noted that the incidence of PPI caused by MRSA has been rated 0.1% after THA and 0.17% in TKA [90].
The comparison between systemic administration of antibiotics and the use of antibiotic-loaded cement as PPI prevention has resulted inconclusive for a long time [56]. A favorable effect by adding antibiotics to the bone cement has been reported in the literature [91]. A cost-effectiveness study [51], with a level II of SE, has reported that antibiotic-impregnated bone cement in primary THA is cost-effective avoiding revision due to infection when the cost of revision is more than 3.5 times the cost of primary THA; when antibiotic cement cost less than US$650; when the utility of revision is lower than 70%, figure close to the quality of life after primary THA; when the risk of revision due to infection is higher than 1.7; and in patients younger than 71 years. It may become the dominant strategy, less costly/more effective, when cost of revision for infection is more than 7.3 times the cost of primary THA; when antibiotic cement cost is less than US$400; when the relative risk of revision due to infection is higher than 2.4; and in patients less than 46 years of age. The estimated cost of a 40-g packet of antibiotic-impregnated bone cement at the authors’ institution was approximately (in 2002) US$365 while standard bone cement cost was approximately US$65 in 2002. Two packets of cement are used on the average, resulting in an additional cost of US$600 per primary THA in 2002. The infection rate assumed in the model was 0.7% over ten years using standard bone cement and 0.4% with antibiotic-impregnated bone cement. A higher risk of infection from baseline makes the option of using antibiotic-impregnated bone cement even more cost-effective.
Three studies dealt with prophylaxis in high-risk groups [45, 50, 80]. One was a cost-identification analysis [45] and two were cost-effectiveness analyses [50, 80].
Considering patients with high-risk to have haematogenous PPI secondary to recurrent skin lesions, ulceration and infection, a cost analysis of prophylaxis with antibiotics to prevent infection of TKA was performed. Assuming a risk of PPI in 7.5% of these cases, an effectiveness of antibiotics to avoid PPI of 85%, a 3 g daily intake of flucloxacillin or cephalexin for one year and an additional cost of 1988 US$42,360 in case of PPI, the cost saved per infection prevented was (in 1998) US$16,532 with flucloxacillin and (1988) US$18,907 with cephalexin. The prevention of 85% of 28 cases of haematogenous PPI secondary to skin lesions among the 12,000 TKA performed in Sweden 1975-85 period time, would save US$449,984 [45].
A screen-and-treat strategy of Staphylococcus aureus carriers before TJR could be a simple, safe, and cost-effective intervention to reduce the risk of PPI. Empirical S. aureus decolonization with nasal mupirocin for patients undergoing TJR should be considered. Mupirocin inhibits bacterial protein and RNA synthesis and is active against MRSA and methicillin-susceptible S. aureus (MSSA) as well as other gram-positive and some gram-negative bacteria. The rate of nasal colonization with MRSA in patients who will undergo TJR is 3.3% [89]. It is applied intra-nasally twice daily for 5 days and is associated with an S. aureus eradication rate of 83% over the short term. In a cost-effectiveness study performed in 2011, level II of SE, the sensitivity analysis sustains the procedure as favorable even if the cost of mupirocin was over US$100 and the cost of PPI management ranged between US$26,000 and US$250,000. The current cost of mupirocin is US$6.23. Treating all patients appears to be the best strategy when the prevalence of carriers of S. aureus and surgical infection is at acceptable levels and when the prevalence of mupirocin-resistant strains is high.
The treat-all strategy is dominant across all potential ranges of utility of life after TJA septic revision surgery [50]. In 2011 also, another study dealing with the same issue determined the costs saving of this program when it resulted in 10% reduction of relative septic revision rate, with an average cost of septic revision greater than US$70,000 (Fig. 2) [80]. An algorithm of screen-and-treat strategy to PPI has been proposed [80].
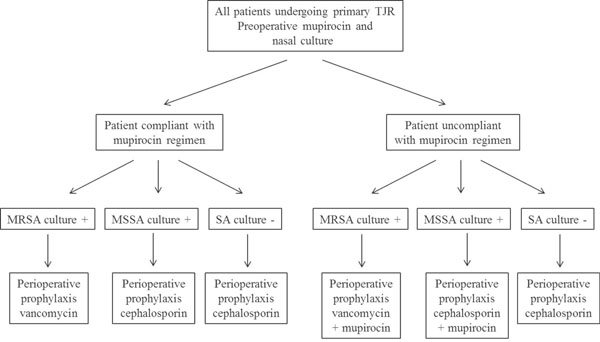
Algorithm of screen-and-treat strategy to PPI [80].
Lavage of the surgical area with 0.35% Betadine, a povidone-iodine topical antiseptic with bactericidal activity against multiple pathogens, including MRSA [47], significantly lowers the incidence of PPI from 0.97% to 0.15% [92]. The Betadine used is a sterile, single-dose pre-pack with a cost of 2012 US$ 1.11, resulting then in a cheap, easy and fast to use, safe, and effective prophylactic agent.
While antibiotic prophylaxis seems the most cost effective method to prevent PPI, the benefits of using a cleaner air in the operating theatre appear to be worthwhile too, even when considered solely in terms of hospital costs [68]. Cost-effectiveness of laminar airflow, ultraclean air systems, body exhaust suits and surgical enclosure, has been evaluated. The cost of a laminar airflow system into a new operating room ranged from US$60,000 to US$90,000 in USA in 2010. The estimated cost of treatment of a single PPI is higher than that [54]. It must be kept in mind that the incidence of PPI was reduced from 2% to l% when using an ultra clean air system, and greater (from 1.3% to 0.3%) when using occlusive clothing [93, 94].
The cost of prophylaxis with systemic antibiotics, locally administered antibiotics, exhaust suits, or surgical enclosure, alone or in combination, is expressed in Table 4 [76]. Parenteral antibiotic prophylaxis alone always costs less on average than any other method, regardless of the number of arthroplasties performed per year. The total cost of prophylaxis and reoperation for PPI could be minimized just by giving parenteral antibiotics. The combination of systemic and local antibiotics would be a cost effective method of reducing PPI rate in hospital departments that performed 100 arthroplasties or less each year. In these departments, the rate of reoperation was nearly the same using gentamicin impregnated bone cement alone or an ultraclean air system in combination with exhaust-ventilated suits, being the former method the less expensive of the two. For departments that performed more than 100 TJR a year, operating in a surgical enclosure with exhaust ventilated suits could reduce the cost of PPI reoperation to a minimum, but the combination of parenteral antibiotics and surgical enclosure would be the most attractive means of prophylaxis from the social point of view. The use of parenteral antibiotics and an ultraclean air system was less expensive than the combination of parenteral and local antibiotic prophylaxis when more than 130 operations were performed each year [75].
Intraoperative bacterial contamination does not mean infection, but an intraoperative positive culture represents an indubitably risk of postoperative PPI [95]. It is imperative to prevent intraoperative contamination and to identify which cases have become contaminated to act in consequence and eradicate the contaminating germs, applying a different regimen on the basis of positivity or negativity of culture performed intraoperatively. From a detailed study was concluded that a combination of systemic and behavioral changes in the operating room, an airflow system among them, significantly decreased the incidence of intraoperative bacterial contamination, and subsequently decreased the incidence of PPI. With behavioral changes such as guidelines for patient work up, use of body coverage, and restricting activity in the operating room, and a new laminar airflow system, the intraoperative contamination decreased from 15% to 5%.
In primary TJR, there was no significant effect of culture result on total costs, while in revision patients with positive cultures generated significantly higher costs than patients with negative cultures. The costs caused during the first three months after operation were substantially higher than those produced in the next nine months. Cost of hospital and supplemental admissions, cost of physiotherapists and homecare, and cost of antibiotics were substantially higher. Mean cost of patients with positive culture outcomes was considerably higher for all the conducted sensitivity analyses, being most evident in the cases of revision [64].
To finish this section, it must be said that a thoroughly systematic search in 2010 [84] regarding the medical effectiveness, cost effectiveness, and ethical, social, and legal aspects related to using interventions to prevent PPI after TKA, failed to find high-level evidence for the effectiveness of different interventions. Most of them were recommended on the basis of results from studies of low quality. A high level of evidence on the effectiveness of systemic antibiotic prophylaxis in TKA was missing because the used information was transferred from THA, and no evidence was found for differences in the effectiveness between various antibiotics. There are strong hints for the effectiveness of antibiotics in cement in addition to systemic antibiotic prophylaxis, but evidence of the effectiveness may be accepted only for operating rooms without clean-air measures. In conclusion, the cost effectiveness of different interventions to prevent infections in TKA remains unclear.
Results of Economic Studies on Diagnosis of Periprosthetic Infection
The diagnosis of PPI is imperative in order to determine the appropriate management of patient in need of revision surgery because treatment, prognostic and outcome may differ depending on whether the arthroplasty is infected or not. However, diagnosis of PPI is often challenging, and a wrong diagnosis will lead to treatment failure, increased morbidity and added costs to the healthcare system.
A good diagnostic tool must have high sensitivity, high negative predictive value, and be cost-effective. In this sense, taken into account the data of Austin et al. [43], the combination of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in the diagnosis of PPI of TKA had a sensitivity, specificity, positive predictive value, and negative predictive value of 0.96, 0.56, 0.58, and 0.95 respectively. The mean ESR and CRP of the infected patients were 85 mm/h and 110 mg/L, respectively, and of the non- infected patients were 22 mm/h and 7 mg/L, respectively. Cost of each one of these tests was US$33 and US$46 respectively in 2008. The disadvantage is that these tests are relatively nonspecific and can be elevated in many clinical conditions, providing a large number of false positives. For that reason, these simple serologic tests must be used as screening but not confirmatory tests. In that context, an aspiration for cell count, a differential count of neutrophils, and culture would be a good combination to be performed in patients in whom the PPI is suspected based on clinical or laboratory findings. The charges for all these tests would be only US$200.
Similar conclusions to those reached for TKA in this study were attained by Spangehl et al. for THA [96]. The determination of leukocyte esterase in the synovial fluid using a standard chemical test strip has a sensitivity, specificity, positive predictive value, and negative predictive value of 0.93, 0.86, 0.72, and 0.97 respectively for infection of TKA. It can yield a result within one to two minutes and the test strips are so inexpensive that a three-pack can be ordered for less than US$2.00 [72]. Further confirmation of utility of this diagnostic test is needed.
Comparing the cost effectiveness of preoperative joint aspiration culture and anuclear medicine study with technetium sulfur colloid and indium-111, to evaluate THA PPI, the sensitivity, specificity, positive predictive value, and negative predictive value were respectively 0.92, 0.91, 0.54, and 0.99 for the former and 0.86, 1.00, 1.00, and 0.92 for the latter [81]. The cost of hip aspiration was approximately 20% as much as nuclear medicine study [43, 81]. Hip aspiration is then an accurate and cost-effective method of evaluating the potentially infected hip prosthesis.
Results of Economic Studies on Treatment of Periprosthetic Infection
Among the 25 studies included in this section, there are only two with a high level of quality [55, 72]. Most of the remaining 23 papers are cost-identification studies with a low level of SE.
The treatment of patients with PPI is associated with significantly greater resource utilization compared with patients who have primary or aseptic revision of TJR, with substantial economic burden for patients, payers, hospitals, physicians, and society. PPI often needs multiple reoperations, prolonged use of antibiotics, long rehabilitation, and frequent follow-up visits. Revision procedures for PPI are associated with significant higher number of hospitalizations, days in the hospital, number of operations, longer operative time, more blood loss, length of antibiotic therapy, number of radiographic examinations, and total outpatient visits during the twelve-month period following the index procedure, and a higher number of complications (Table 5). In general, in case of an infected TKA, these parameters and cost were 3 to 4times that of a primary TKA and more than twice that of an aseptic revision [57]. Sculco, in 1993, estimated an average cost of US$50,000 to $60,000 per case of infected THA [78]. Patients with PPI TKA required 8.49 more days of hospitalization than those without infection. A total hospital charges model indicated that patients with PPI TKA used US$928 more in health care than did patients without infections [82]. The number of hospitalizations for PPI increased in USA from 74.4 per 100,000 hospitalizations in 1997 to 107 per 100.000 hospitalizations in 2004 with an increase in the annual adjusted diagnostic-related group cost from US$195 million to US$283 million [58].
Average of Number of Hospitalizations, Days in the Hospital, Number of Operations, Operative Time, Blood Loss and/or Transfusion, Length of Antibiotic Therapy, and Total Outpatient Visits Per Patient, in PPI Compared with Primary Arthroplasty ℗ or Aseptic Revision ®
| Author [Reference] Date | Number of Hospitalizations | Days in Hospital | Number of Reoperations | Operative Time (Min.) | Blood Loss/ Transfusion | Days in ICU | Length of Antibiotics | Number Rx Examinations | Outpatient Visits |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| TJR (THA + TKA) | |||||||||
|
|
|||||||||
| Peel [74] † 2011 | 2 | 31.6 | 3.3 | 100 | 0 | 9.9 | |||
| ℗ 1 | ℗ 7.9 | ℗ 0.07 | ℗ 95 | ℗ 0 | ℗ 2.9 | ||||
|
|
|||||||||
| THA | |||||||||
|
|
|||||||||
| Kurz [65] 2004 | 9.7 | ||||||||
| ℗ 4.1 | |||||||||
| ® 5.4 | |||||||||
|
|
|||||||||
| Bozic [46] 2005 | 3.6 | 28.2 | 3.7 | 278 | 2082 cc | 54.6 | |||
| ℗ 1.2 | ℗ 6.2 | ℗ 1 | ℗ 177 | ℗ 449 cc | ℗ 17.2 | ||||
| ® 1.2 | ® 8.1 | ® 1.4 | ® 299 | ® 1569 cc | ® 28.2 | ||||
|
|
|||||||||
| Monge Jodra [70] 2006 | 53 | ||||||||
| ℗ 17 | |||||||||
|
|
|||||||||
| Iribarren [86] 2007 | 54.8 | 0.83 | 1.1 | ||||||
| ℗ 12.5 | ℗ 0 | ℗ 0 | |||||||
|
|
|||||||||
| Klouche [63] 2010 | 30.6 | 0.9 | |||||||
| ℗ 7.5 | ℗ 0.05 | ||||||||
| ® 8.9 | ® 0.2 | ||||||||
|
|
|||||||||
| Kurz [66] 2012 | 9.5 | ||||||||
|
|
|||||||||
| TKA | |||||||||
|
|
|||||||||
| Bengston [45] 1989 | 145 | 3.3 | 1328 | 12 | 16 | ||||
| ℗ 14 | ℗ 1 | ℗14 | ℗ 3 | ℗ 3 | |||||
|
|
|||||||||
| Hebert [57] 1996 | 2.3 | 32.1 | 3.1 | 410 | 4.6 EC | ||||
| ℗ 8.7 | ℗ 122 | ||||||||
| ® 12.8 | ® 225 | ||||||||
|
|
|||||||||
| Kurz [65] 2004 | 7.6 | ||||||||
| ℗ 3.9 | |||||||||
| ® 4.2 | |||||||||
|
|
|||||||||
| Lavernia [67] 2006 | 16.1 | ||||||||
| ® 6.6 | |||||||||
|
|
|||||||||
| Dal-Paz [52] 2010 | 28.7 | 1.9 | 1 | ||||||
|
|
|||||||||
| Oduwole [71] 2010 | 39 | ||||||||
| ® 15.5 | |||||||||
|
|
|||||||||
| Haenle [85] 2012 | 48.2 | 4.1 | 64 | 4.5 EC | 2.5 | ||||
| ℗ 13.4 | ℗ 1 | ℗ 82 | ℗ 0.6 | ℗ 0 | |||||
|
|
|||||||||
| Kurz [66] 2012 | 7.2 | ||||||||
Treatment costs per patient vary dramatically according to the different studies, country and dates of achievement (Table 6), and with the specific type of applied treatment (Tables 7-9). The cost of hospital stay was in all cases the more relevant component in absolute value. In Durham Regional Hospital (North Carolina, USA), in the 90s’, the total direct cost of hospitalization was estimated an average of US$8206 for infected TJR vs US$5492 for uninfected arthroplasties [62]. In the KrakówJagielloński University (Poland), in 2005, the direct cost of hospitalization for infected TJR reached US$37,903 and the cost of antibiotic treatment was US$11,067 [87]. In the Hospital of the University of Lund (Sweden), the cost of hospitalization in 1988 was US$2530 for primary TKA vs US$33,663 for infected TKA, the cost of operation was US$3684 and US$10,411 respectively, and the cost of antibiotic therapy was US$65 and US$3778 respectively [45]. Detailed all acute inpatient, rehabilitation and outpatient costs for the treatment of infected TJR, in St Vincent’s Hospital, Melbourne (Australia), from 2008 to 2010 [74]; staff, pharmaceuticals, medical supplies, implants, medical areas and equipment (operating room, anesthesia, intensive care, physiotherapy, radiology, laboratory, pharmacy), general services (administrative services, maintenance, catering, laundry), devaluation of equipment, and financial costs for the treatment of infected THA, in two French hospitals, in 2006[63]; and the hospital total cost of treatment of infected TKA, in Rostock (Germany), from 2004 to 2007 [85], were collected and shown in Tables 8 and 9.
Estimated Average Cost (and Range) in 2012 Adjusted Currencies and Normalized to US$, of Primary, Aseptic and Septic Revision THA/TKA
| Author [Reference] Date | THA | TKA | ||||
|---|---|---|---|---|---|---|
| Primary℗ | Revision Aseptic® | Revision Septic | Primary℗ | Revision Aseptic® | Revision Septic | |
| Bengston [45] † 1989 | 10,534 | 77,902 x7.4℗ | ||||
| Bengston [44] † 1993 | 13,677 | 98,761 x7.2℗ | ||||
| Hebert [57] ‡ 1996 | 46,542 | 67,891 | 158,407 x3.4℗/x2.3® | |||
| Azanza [83] † 2001 | 7617 | 16,874 x2.2℗ | 7894 | 18,742 x2.3℗ | ||
| Kurz [65] ‡2004 | 47,250 | 57,648 | 86,176 x1.8℗/x1.5® | 43,248 | 51,882 | 68,907 x1.6℗/x1.3® |
| Bozic [46] † 2005 | 38,535 | 65,488 | 184,566 x4.8℗/x2.8® | |||
| Cummins [51] (from [46]) | 27,655 | 122,818 x4.4℗ | ||||
| Evans [54] (from [46]) | 163,891 | |||||
| Courville [50] (from [46]) | 107,491 | 120,920 | ||||
| Knobben [64] * 2006 | 16,227 (8422-76,142) | 57,752 | 74,512 (64,541-84,626) | 17,684 (9110-33,024) | 86,214 | |
| Lavernia [67] ‡ 2006 | 79,009 | 125,914 x1.6® | ||||
| MongeJodra [70] ‡ 2006 | 9970 | 31,084 x3.1℗ | ||||
| Iribarren [86] ‡ 2007 | 2700 | 7081 x2.6℗ | ||||
| Dal-Paz [52]◊ 2010 | 3021 | |||||
| Klouche [63] † 2010 | 12,639 | 17,373 | 45,564 x3.6℗/x2.6® | |||
| Oduwole [71] ‡ 2010 | 20,731 | 31,577 x1.5® | ||||
| Romanò [77] ‡ 2010 | 37,154 | 82,512 x2.2® | ||||
| Haenle [85] ‡ 2012 | 9320 | 34,086 x3.6℗ | ||||
| Kurz [66] ‡ 2012 | 93,600 | 74,900 | ||||
‡ Total hospital costs.
◊ Direct cost hospital stay, laboratory tests, imaging examinations, and surgical procedures performed.
† Total hospital costs + total outpatient costs (out of social costs).
* Inpatient care + general health care + surgery + medication + outpatient care + informal cares + out-off-pocket costs + productivity losses.
℗ Primary.
® Revision.
Estimated Average Cost (and Range) in 2012 Adjusted Currencies and Normalized to US$, of Different Options of Treatment of PPI
| Author [Reference] Date | Debridement and Retention | One-Stage Revisión ① | Two-Stage Revisión ② | Resection Arthroplasty | Arthrodesis | Amputation |
|---|---|---|---|---|---|---|
| TJR (THA + TKA) | ||||||
| Peel [74] † 2011 | 75,661 | |||||
| THA | ||||||
| Fisman [55] † 2001 | 74,015 | 70,634 | ||||
| Klouche [63] † 2010 | 43,586 | 75,737 x 1.7 ① | ||||
| TKA | ||||||
| Hebert [57] ‡ 1996 | 150,984 | 121,866 | 101,346 | 347,789 | ||
| Lavernia [67] ‡ 2006 | 133,970 | 134,670 | 113,575 | |||
‡ Total hospital costs.
† Total hospital costs + total outpatient costs (out of social costs).
① One-stage revision.
② Two-stage revision.
Cost of Non-Infected TJR and Debridement and Retention for Treatment of Infected TJR (Data from Peel et al. [74])
| Non-Infected TJR | Infected TJR D&R | p | |
|---|---|---|---|
| Total inpatient | 22,688 | 57,494 | .001 |
| Medical | 1732 | 9117 | .001 |
| Nursing | 7830 | 28,140 | .001 |
| Operatingroom | 11,173 | 18,977 | .001 |
| Implants | 7468 | 8336 | .3 |
| Intensivecareunit | 0 | 0 | 1.0 |
| Alliedhealth | 1562 | 3707 | .001 |
| Medical imaging | 64 | 278 | .001 |
| Pathology | 188 | 1710 | .001 |
| Pharmacy | 331 | 2388 | .001 |
| Hospital at home | 469 | 1624 | .02 |
| Total outpatient | 377 | 4426 | .001 |
| Medical | 23 | 901 | .001 |
| Nursing | 278 | 442 | .03 |
| Alliedhealth | 0 | 44 | .002 |
| Medical imaging | 0 | 120 | .001 |
| Pathology | 0 | 146 | .001 |
| Pharmacy | 0 | 1846 | .001 |
| Total emergency | 0 | 553 | .001 |
| Total costs | 24,073 | 75,661 | .001 |
| Concept | THA | TKA | |||
|---|---|---|---|---|---|
| Primary | Revision Aseptic | Revision Septic | Primary | Revision Septic | |
| Preoperative office visits | 64 | 64 | 64 | ||
| Laboratory examinations | 222 | 222 | 222 | 119 (1.28%) | 362 (1.07%) |
| Histological examinations | 6 (0.07%) | 151 (0.44%) | |||
| Microbiological examinations | 0 | 151 | 151 | 20 (0.22%) | 180 (0.53%) |
| Imaging examinations | 104 | 201 | 638 | 229 (2.46%) | 335 (0.98%) |
| Blood products | 45 (0.61%) | 680 (2.00%) | |||
| Antibiotics | 290 | 344 | 1178 | 7 (0.08%) | 702 (2.05%) |
| Pharmaceuticals | 131 (1.41%) | 427 (1.26%) | |||
| Medical supplies | 172 | 204 | 3839 | 1767 (18.96%) | 6115 (17.94%) |
| Implants | 2531 | 2866 | 2607 | 2941 (31.57) | 7971 (23.39%) |
| Operation theatre | 2987 | 4310 | 4060 | 371 (3.98%) | 966 (2.83%) |
| Anesthesia | 1105 (11.86%) | 2879 (8.45%) | |||
| Intensive care unit | 1104 | 0 | 4170 (12.23%) | ||
| General ward | 2611 | 3094 | 13,927 | 2563 (27.52%) | 9146 (26.83%) |
| Rehabilitation | 288 | 343 | 543 | ||
There were also notable differences between the costs generated by the use of antibiotics during hospitalization and costs of administered antibiotic in outpatients. In TKA infection, the average length of treatment in hospital was 157 days against the 850 total days of outpatient treatment. In 1999, in Navarra (Spain), the cost of antibiotic therapy during the hospital stay was US$3140 per patient that is US$20 per patient per day of hospitalization. The cost of outpatient antibiotic therapy was US$935per case, representing US$1.1 each day [83]. The effort to reduce the inpatient treatment, even if the outpatient treatment is long, has a notable impact on the total cost. In this sense, the trend of treatment of PPI would be directed toward the use of oral antibiotic therapy in outpatient, reducing stays essentially to those related to surgical procedures.
Surgical options for treatment include debridement and retention of prosthesis (D&R), one- or two-stage exchange (OSE and TSE), resection arthroplasty, arthrodesis and amputation. Treatment alternatives must be selected based on specific criteria as the responsible pathogen, the patients’ immunologic status, the chronicity of the infection, and the stability of the implant. The success rates in eradication of PPI were below 50% with D&R of TJR in retrospective series, but over 70% in the prospective modern studies with optimal use of antibiotics [97, 98]. When prosthetic components are mechanically stable, symptoms may last three weeks or less, the soft tissues are in good condition, and an agent active against the specific germs is available, an adequate D&R of implant achieves an 82%-100% cure rate of infection after three to six months of systemic therapy with ciprofloxacin and rifampin as compared with a 58% cure rate with ciprofloxacin and placebo [98]. Control of PPI with D&R and adequate antibiotic regimen has been reported in 87%-89% of cases recently [99, 100]. It has been suggested a total antibiotic treatment duration of three months for infected THA and of six months for infected TKA [98]. There is a risk of failure with this strategy after stopping antibiotics, but lengthening antibiotic therapy may simply postpone, rather than prevent, failure [99].
Exchange arthroplasty is supported by many studies but has a higher rate of surgical morbidity and is more expensive than D&R. In a systematic review of longitudinal studies with series of more than 50 patients, the success rate to eradicate PPI in THA was reported between 73.6% and 96.7% for OSE and between 87.7% and 95.1% for TSE depending on the different authors. The random-effects analysis showed the rates of re-infection after one- and two-stage revisions were 10.56% and 8.71% respectively [31]. In a recent meta-analysis, re-infection occurred with an estimated absolute risk of 13.1% with OSE and 10.4% with TSE [101].
The use of antibiotics added to the bone cement in OSE procedure led to 88%-93.7% eradication of THA infection [102]. Likewise, a cementless prosthesis may achieve 92% rate of infection control in OSE [103] or in TSE [104-106]. As just noted, TSE has a high rate of success in controlling PPI, but the cost to the patient and the healthcare system is greater because the two operative procedures required. There is no suggestion in the published studies that one- or two stage methods have different reinfection outcomes. If re-infection rates are similar, a single major surgery, with reduced length of hospitalization, avoiding a period without a functional arthroplasty, would be preferable [31].
In a decision-analysis, assuming that success of a given procedure was a period greater than 2 years without additional surgery, OSE might be the best solution for an acute THA infection and lead to the greatest health-related quality of life, whereas the failure rate of D&R is greater than 40% and the success of OSE is 66% or greater. With less than38% success ofD&R and less than 69% success of OSE, TSEmight result in the greatesthealth-related quality of life [107]. However, this might be highly unlikely because the figures of D&R and OSE success are very low compared to those achieved in most series. In one-year and ten year decision models, OSE was associated with greater benefit in terms of quality-adjusted life years than TSE. In the one-way sensitivity analysis, equivalence between the procedures was achieved when the assumed re-infection rate in the OSE arm was 59.8% in the one-year model and 61.2% in the ten-year model, favoring OSE since infection rates in those ranges are fourfold to fivefold higher than what have been reported in the literature [108].
The economic effects of OSE and TSE differ considerably. Although OSE may require a long hospital stay to administrate parenteral antibiotic therapy, the main determinant of cost is the requirement for additional surgery in TSE, with a cost 1.7 times more than OSE [63]. A short interval until re-implantation (two to four weeks) could allow both procedures to be performed during a single hospitalization [109].
The clinical and cost effectiveness of D&R and TSE, with a median time to re-implantation of 2 months (range 1-12 months), in 65-year-old and frail 80-year-old patients with infected THA has been compared. Patients who underwent initial D&R were subjected to more additional operations than those who had initial exchange arthroplasty (3.2 vs 2.4 on average). In all cohorts, initial TSE provided a higher rate of infection-free survival than initial D&R. However, the quality-adjusted life expectancy associated with D&R was greater than with TSE when old and frail population was considered. Incremental cost-effectiveness ratio (ICER) of D&R compared with initial exchange arthroplasty was (in 1999) US$19,700 per QALY gained for 65-year-old men, US$21,800 per QALY gained for 65-year-old women, US$500 per QALY for 80-year-old men and US$8200 per QALY for 80-year-old women. Initial D&R became cost-saving strategy relative to exchange arthroplasty when age at initial diagnosis of infection was over 80 years, when indirect and patient time costs were included in the analysis, and when the annual rate of infection recurrence after debridement was less than 19%. Even if the annual relapse rate after exchange arthroplasty was as low as 0.6%, initial D&R remained cost-effective for patients over 80 years. The authors conclude that debridement and retention are reasonable strategies for treatment of PPI in patients over 80 years, staphylococcal or streptococcal infection, and well-fixed prosthesis.
However, some caution should be necessary when the results are applied to S. aureus PPI with delayed diagnosis and treatment. The wide variability in the cost-effectiveness ratios indicates a significant risk of debridement and retention not being cost-effective on the contrary of that sustained by the authors [55].
For TKA, the efficacy of the different approaches to heal PPI is 20% for antibiotic therapy alone, 24% for debridement of soft tissue, 50% for resection arthroplasty, 76% for exchange arthroplasty, 90% for arthrodesis, and 100% for amputation [45]. In a systematic search of the literature about infected TKA, the overall success rate of PPI eradication was 73%-100% after OSE and 82%-100% after TSE, with 12-122 months follow-up [110]. The clinical outcome (knee scores and range of motion) of OSE was not different from that of TSE.
There are some pejorative factors increasing the burden that a PPI supposes. The number of hospital stays, readmissions and emergency department visits, the length of stay, the complexity of procedures and antibiotic therapy, the more extended course of recovery, and costs, are significantly higher in the MRSA infections compared to MSS cases (Table 10). Patients infected with methicillin resistant organisms are 4 times more likely to fail treatment [73].
Total Number of Visits, Days of Hospital Stay, and In-Hospital Cost Per Patient and Per Procedure Treating PPI Caused by MRSA and MSSA (Data from Parvizi et al. [73])
| MRSA | MSSA | p | MRSA/MSSA Quotient | |
|---|---|---|---|---|
| Visits per patient | 3.17 | 2.68 | .02 | 1.2 |
| Days in hospital per patient | 38.13 | 21.38 | .0001 | 1.8 |
| In-hospital cost (2009 US$) | 107,264 | 68,053 | .0001 | 1.6 |
| Length of Stay (Days) Per Procedure | ||||
| Debridement and retention | 15.91 | 7.87 | .0001 | 2.0 |
| One-stage exchange | 10.67 | 6.73 | .0397 | 1.6 |
| Reimplantation | 8.25 | 5.60 | .0049 | 1.5 |
| Resection arthroplasty | 12.84 | 9.42 | .0039 | 1.4 |
| Cost (2009 US$) Per Procedure | ||||
| Debridement and retention | 32,720 | 18,734 | .001 | 1.7 |
| One-stage exchange | 36,606 | 25,886 | .033 | 1.4 |
| Reimplantation | 35,022 | 26,775 | .0105 | 1.3 |
| Resection arthroplasty | 30,387 | 23,495 | .0199 | 1.3 |
Revision of infected arthroplasties might be facilitated by certain methods and accessories. A cost-effectiveness analysis of a saline coupled bipolar sealing system to reduce intraoperative bleeding, allowing the surgeon to work more quickly and minimizing blood loss, has been performed. There was a significant decline in intraoperative blood loss and in operative time when the bipolar sealing was compared with a conventional electrocautery. The use of the saline coupled bipolar sealing system cost approximately US$500 per case in 2011.
From the perspective of hospitals or surgical practices running their own operating rooms, the net financial impact ranges from a cost of US$5.09 to savings of US$36.15 per case. From the perspective of physician groups renting operating room space, the 24 minutes gained in operative time are supposed to bring a reduction in fees of approximately US$1794.91 per case, and accounting for the cost of the device, net savings may be estimated US$1294.91 per case [49].
The use of an antibiotic-loaded spacer in the TSE treatment of infected THA provides better infection control with good functional results and is superior to treatment in two-stages without a spacer. The recurrence of infection was significantly higher without spacer (33.3% vs 10.5%). The use of a spacer increased the surgical time of first stage by 40.1 minutes, but reduced the mean duration of the second surgical stage in 1 hour because re-implantation is easier, finding readily the surgical planes, identifying well the bone structures, and building adequately the bed to insert the prosthesis. The stay in the intensive care unit after the second surgical stage was shorter when using a spacer (average, 1.4 days vs 4.1 days). Patients without a spacer stayed in hospital almost twice as long as patients with a spacer because a period of skeletal traction is mandatory to allow healing of the soft tissues maintaining as much as possible the length of extremity. The shorter extent of operating time, hospital stay, and intensive care unit stay, must definitely lead to a lower cost when using a spacer in TSE than without the spacer [111].
Another way to cost savings in the treatment of PPI is to use the liquid form of gentamicine mixed to the bone cement fixing the prosthetic components or filling the cement spacers employed in TSE. It is the most widely and readily available antibiotic for mixing to the bone cement, and much less costly (US$4 for a 480-mg dose) than tobramycin (US$120-310 per 1.2-g dose) and the powdered form of gentamycin, at least as expensive as tobramycin [60, 79]. The limitation of use of liquid gentamicin in bone cement fixing prosthesis is the decrease of mechanical properties of cement produced, but this is irrelevant for the temporary cement spacers [79]. If tobramycin is replaced by the use of liquid gentamicin in bone cement spacers, an annual antibiotic cost saving of US$7,400,000 could be achieved in the United States [79].
From the current literature it emerges a certain consensus that complete cost coverage ofPPI treatment is not feasible in most healthcare systems. An estimated average loss of approximately $15,000per case for the total group as a whole of patients treated for infected TKA, and $30,000 per case per Medicare patient in USA in 1993 [57], and US$7745 per case in Germany in 2007 [85]. Even more, the inflation decreased the value of estimated mean reimbursement per hospitalization for PPI in USA from 2004 US$9746 in 1997 to US$8719 in 2004 [58]. The lack of incremental reimbursement for these procedures discourages physicians and hospitals to treat patients with PPI [46]. Reimbursement to both hospitals and physicians should be more accurate and reflect really the magnitude of resources consumed by these patients.
DISCUSSION
Determining the treatment that best controls infection, minimizing patient morbidity and mortality, and with the less cost possible may offer the best solution to the problem. Among protocols and techniques currently used to reduce the incidence and to treat PPI, no clear best option exists. The resource allocation and financial costs of treating PPI in orthopedic surgery can often rise 3-13 times more than the cost of the index procedure, thus making PPI an ideal target for cost-effective solutions in a value-driven healthcare model [65]. The recommendations set out by different authors [109, 112], and demonstrated useful when choosing the method of treatment of PPI [113], must be tempered in view of results ofthese economic studies.
There are only few high-quality studies dealing with an accurate evaluation of cost-effectiveness of prevention, diagnosis or treatment of PPI. The lack of level-I evidence studies regarding interventions in PPI has made more difficult to perform high-quality cost-utility analyses. It is obvious the difficulty and ethical concerns to perform randomized studies in this field, especially as it relates to treatment procedures. The number of patients needed to carry on correctly these studies is another concern. It has been estimated that 7,000-14,000 patients would be needed to demonstrate a 20% reduction in infection rate if the baseline infection rate were 5% [114]. A power study assuming a 1.5% to 2.0% infection rate would require on the order of 10,000 patients to determine the effect of any one independent variable, with power greater than 80% [54]. In order to show a 50% reduction in an infection rate of 2%, for example, at the 5% significance level and 80% power, over 2300 patients would be required in each treatment arm [56]. Multiple variables would require at least 70,000 patients [54]. For these reasons, should not be undertaken or supported further under-powered trials dealing with such issues. Given the low PPI rates, it may not be cost-effective to carry out mega-trials in this area. It is necessaryto analyze risk factors to identify high-risk groups on whom profitable high-quality studies of new or additional prophylactic, diagnosis, or treatment measures could be performed with sufficientpower to achieve a statistically significant difference.
Either materials or methodology, or both, were incompletely described in a considerable number of examined studies. The availability and access to all clinical and economic data [83], and their accurate and thorough gathering and treatment [115], following sound economic-analysis principles, are essential but infrequent. The review of the literature assessing the adequacy of publications on the economics of prevention, diagnosis or treatment of PPI has shown that the majority lacked the standards of quality required for these studies. The information specified in most of them should be interpreted with caution due to null or incomplete account of functional outcomes, quality of life, non-medical direct and indirect costs, costs/benefits, and the outdated reported data. There are important limitations relative to the collection and comparison of cost allocation data, particularly on an international level.
Most of the studies in this field have estimated charges or costs of management of PPI assuming partially the real price of actions. The direct medical costs, length of hospitalization, and total hospital costs were the most frequently considered parameters as indicators to evaluate resource utilization [52, 57, 85], while the outpatient charges, the costs associated with re-treatment of failed treatment, and the indirect costs associated with lost wages and productivity only sometimes were accounted [45, 46, 55, 64]. The use of the direct costs of hospitalization has been suggested as the best method to estimate the costs related to infection treatment, since it not only represents the real costs to the hospital for the items and services used by each patient, but probably underestimates the total resource utilization and also misjudges the total financial and personal impact of PPI on the patients themselves [116, 117].
On the other hand, charges are only a proxy for cost and an inaccurate measure of health-care resource utilization for many reasons, essentially for the fact that the economic basis of charges differs substantially among health-care facilities and geographic locations. Impact on functional outcomes, working and daily activities, quality of life and well-being should also be considered. Thus, the burden on patients of PPI could far exceed the costs usually evaluated in this kind of studies.
All this makes economic analyses of this topic, a difficult, scarce and challenging issue. There is a challenge identifying and selecting economic analyses for review, critically assessing these analyses, and presenting the results of the systematic review [118]. Because of the heterogeneity of the studies and the materials used in them, meta-analytical technique has not been used. Instead, the studies were reviewed descriptively, which limits the objectivity of conclusions and leaves room for interpretative disagreement.
Future studies should attempt to measure the total costs of PPI, including indirect costs incurred by patients and society, and forthcoming costs associated with complications or further interventions, using sound healthcare economic techniques to properly evaluate and assess the true economic and social costs of PPI. Using inexpensive tools that currently exist and applying them in new and innovative ways, is an area of orthopedic research that should be further pursued and that may provide the cost-effective solutions that the current healthcare environment demands.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


