RESEARCH ARTICLE
Expression of Vascular Endothelial Growth Factor on Chondrocytes Increases with Osteoarthritis - An Animal Experimental Investigation
C.O Tibesku*, 1, K Daniilidis2, A Skwara3, J Paletta3, T Szuwart4, S Fuchs-Winkelmann3
Article Information
Identifiers and Pagination:
Year: 2011Volume: 5
First Page: 177
Last Page: 180
Publisher ID: TOORTHJ-5-177
DOI: 10.2174/1874325001105010177
Article History:
Received Date: 15/2/2011Revision Received Date: 4/4/2011
Acceptance Date: 12/4/2011
Electronic publication date: 18/5/2011
Collection year: 2011

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Objective:
To evaluate the expression of VEGF by chondrocytes of hyaline cartilage during the course of osteoarthritis (OA).
Methods:
In 12 white New Zealand rabbits the anterior cruciate ligament (ACL) was resected to create an anterior instability of the knee. In 12 control rabbits only a sham operation without resection of the ACL was done. Four animals of each group were killed at 3, 6, and 12 weeks. The load bearing area was evaluated histologically according to Mankin and by immunostaining for VEGF.
Results:
In the experimental group, histological grades of OA showed a positive linear correlation with the time after surgery. Immunostaining showed an increased expression of VEGF in the control group after 3 weeks, which dropped to normal after 6 weeks. There was no difference in the progression of OA between control and experimental groups after 3 weeks, but a significant difference was seen after 6 (p=0,01) and 12 (p=0,05) weeks. A significant positive correlation between VEGF expression and the histological grade of OA was found (r = 0.767; p<0.01).
Conclusions:
An increase of VEGF expressing chondrocytes occurs during time course of OA.
INTRODUCTION
Osteoarthritis is a degenerative disease of the hyaline cartilage of the articular surfaces, directly related to ageing and mechanical damage [1], resulting in matrix degradation [2, 3]. Several studies of late stage arthritis showed that VEGF is expressed in osteoarthritis [4-6], indicating that angiogenesis might be involved in the pathology of OA. In fact, in severe arthritis [7], angiogenesis has been observed. Little is known about the time depended expression of VEGF during the time course of OA. This study was designed to evaluate (a) the time depending expression of VEGF during the course of OA and (b) possible correlations with histological findings.
MATERIAL AND METHODS
Animals, Surgery and Macroscopic Evaluation
The experiments were performed with permission of the local government (G49/2000) in accordance with National Institute of Health (NIH) guidelines as described earlier [8]. Briefly, twenty eight fully grown, female white New Zealand rabbits were used. In the experimental group (12 animals), the anterior cruciate ligament was resected to create instability of the knee. In the control group (12 animals), only a sham operation without resection of the ACL was done. Four animals of each group were killed at 3, 6, and 12 weeks postoperatively. In addition, four animals (0 weeks) did not undergo any treatment and were killed at the time of surgery. After slaying the animals, both distal femurs were prepared and immediately stored at –80°C. Macroscopic grading of arthritis was done as described previously [8].
Histological Evaluation
Sample preparation and immunostaining were performed as described earlier [8], with some modifications. Briefly, frozen tissue samples were thawed, fixed with 4% paraformaldehyde, decalcificated using buffered EDTA (20%, pH 7.4), dehydrated and embedded in paraffin. Sections (5 µm thick) were cut, mounted on poly-L-lysine coated glass slides, deparaffinized in xylene, and washed. Epitope unmasking was done using proteinase K digestion (DAKO, Germany) for 5 minutes and endogenous peroxidase activity was quenched by treating tissue sections with 3% H2O2 for 10 minutes, non-specific binding was blocked using 3% bovine serum albumin (BSA/TrisHCl), each followed by an excessive washing procedure. Sections thus prepared were incubated with monoclonal mouse IgG antibodies against VEGF diluted 1:50 in Tris/BSA (Calbiochem Clone JH 121) at 4°C over night. Visualization was done using EnVision+ System- HRP with AEC-chromogene (DAKO Envision Systems, Germany, K4001). As control instead of primary antibody, mouse IgG1 with irrelevant specificity (Aspergillus niger glucose oxidase, DAKO, Germany) was used. In addition, sections were incubated without primary antibody.
Image Analysis
Histological sections were photographed (CoolSnap-Pro Color A00J82025, Media Cybernetics, Silver Spring, MD, USA). The cells were counted using the Image-Pro Plus software for Windows, version 4.1 (Media Cybernetics, Silver Spring, MD, USA). The total number of chondrocytes was determined in a hematoxylin and eosin stained section and compared with the number of immunohistochemically stained cells.
Histological grade of OA was evaluated according to Mankin [9] by two independent investigators who were unaware of the source of the specimens. The sections were decalcified by using EDTA which leads to an adapted mankin score which we used in the present study.
Statistical Analysis
Statistical analysis was performed using SPSS 11.0 (SPSS GmbH, Munich, Germany). Student’s t test was used for comparison of scores, and Spearman´s coefficient was calculated for correlations. P values less then 0.05 were considered significant.
RESULTS
Two animals developed postoperative hematomas, first a superficial wound infection treated with a single shot antibiotic, and second animal wound gap re-operated on 2 days after surgery. All four animals were included in the analysis. At the time of death, one animal of group one had intact ACLs and was excluded from evaluation. In total, 54 joints were evaluated.
Histological Grading of OA
According to the Mankin score, the experimental group showed a highly significant increase in the grade of OA in comparison with the control group and the untreated group (Figs.3-6). In the untreated group the mean (SD) grade of OA was 1.75 (1.58). In the control group it was 3.25 (0.71) after 3 weeks, 1 (1.20) after 6 weeks, and 2.25 (1.17) after 12 weeks. In the experimental group, the scores were 7.25(3.62) after 3 weeks, 8 (2.88) after 6 weeks, and 9.83 (3.06) after 12 weeks. The experimental group had significantly higher values than the control group at every point of time (p<0.01). The average histological grade of OA increased with the number of postoperative weeks. It is remarkable that in the control group the histological grading increases within the first three weeks and drops to normal in the following time.
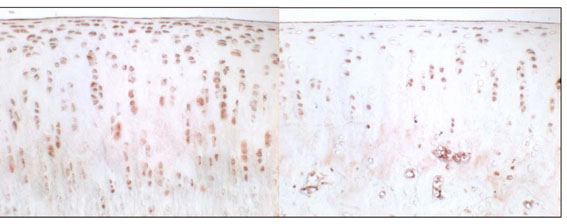 |
Fig. (3). Immunohistochemical staining of VEGF, 3 weeks after sham operation (left side) and 3 weeks after resection of the anterior cruciate ligament (right side). |
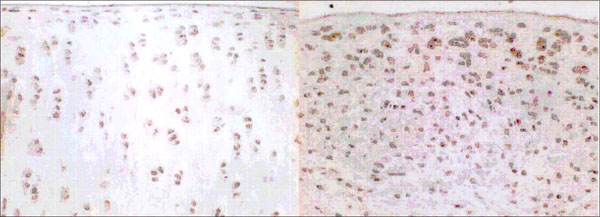 |
Fig. (4). Immunohistochemical staining of VEGF, 6 weeks after sham operation (left side) and 6 weeks after resection of the anterior cruciate ligament (right side). |
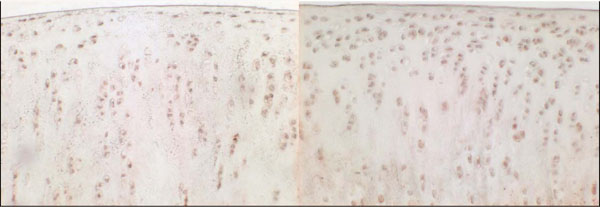 |
Fig. (5). Immunohistochemical staining of VEGF, 12 weeks after sham operation (left side) and 12 weeks after resection of the anterior cruciate ligament (right side). |
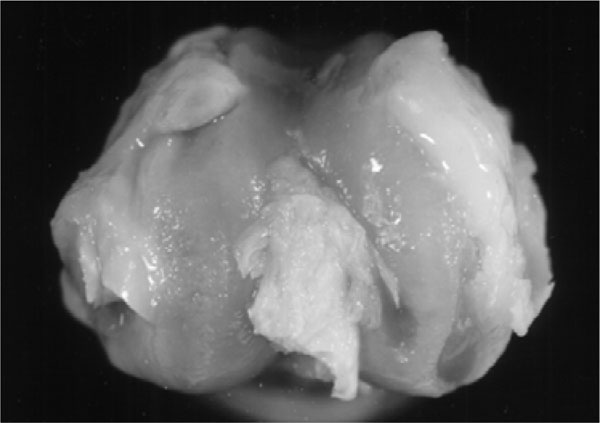 |
Fig. (6). 12 weeks after acl resection showing pronounced osteophytes and exposure of the subchondral cartilage zone. |
Immunohistochemical Staining of VEGF
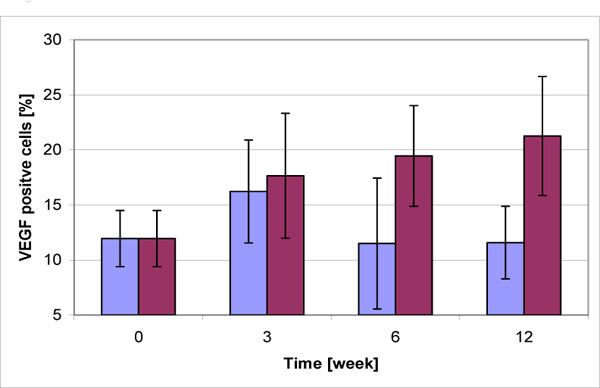
Chondrocytes expressing VEGF were found in the experimental and control groups (Fig. 1). In the control group, the mean percentage (SD) of VEGF positive cells was 16.2 ± 4.7 % after 3 weeks, 11.5 ± 5.9 % after 6 weeks, and 11.6 ± 3.3 % after 12 weeks. In the experimental group, percentages were 17.7 ± 5.7 % after 3 weeks,19.4 ± 4.6 % after 6 weeks, and 21.3 ± 5.4 % after 12 weeks (Fig.1).There was no significant difference between control and experimental groups after 3 weeks (p= 0.594), but a significant difference was seen after 6 and 12 weeks (p=0.01 and p=0.05). It is remarkable that in the control group the percentage of VEGF expressing chondrocytes increases within the fist three weeks and drops to normal in the following time.
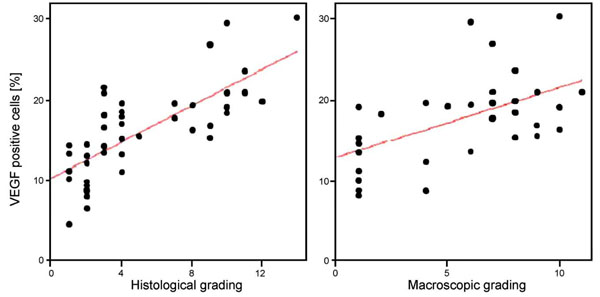
A significant correlation was found between the percentage of VEGF positive chondrocytes and the histological grade (r = 0.767, p = 0.01) as well as the macroscopic grade (r = 0.518, p = 0.02) (Fig. 2). Focusing on osteophyte formation (as part of the macroscopic grade) a positive correlation (r = 0.651; p< 0.01) was detectable. The VEGF staining occurred throughout the cartilage.
DISCUSSION
The present study demonstrates that VEGF is synthesized by chondrocytes of articular cartilage in knees of healthy rabbits. With the development of osteoarthritis due to ACL resection this ratio alters, resulting in an increase of VEGF expressing chondrocytes, correlating with the degree of osteoarthritis. Therefore, the expression of VEGF might be a useful marker of OA. Our data, obtained in an experimental animal model, corresponds with findings of others [4-6, 10, 11] who found that VEGF expression is up regulated in human chondrocytes during late stage OA.
Osteophyte formation and extension of the subchondral growth plate have been observed in OA and involve processes very similar to enchondral ossification, which is highly dependent on VEGF [12]. In our studies, the amount of VEGF producing chondrocytes was positively correlated with the osteophyte formation. A methodological limitation of our study is that we mixed the values of sham and ACLT in the statistic which could influence the present results.
Focusing on the time course of the control group, three weeks after surgery the percentage of VEGF expressing chondrocytes increases although no mechanic instability was induced by ACL resection. This might be due to angiogenic process common in wound healing [13, 14]. Nevertheless, it is remarkable that the histological grading according to Mankin is raised after 3 weeks and drops to normal in the following time, comparable to the percentage of VEGF expressing chondrocytes. This might be interpreted that VEGF alone has a negative impact on the integrity of the articular cartilage as postulated by Pufe [15]. This finding has far reaching consequences; for antiangiogenic therapy might be expedient in the treatment of osteoarthritis. Further studies are needed to evaluate VEGF as a target in osteoarthritis treatment.