RESEARCH ARTICLE
Total Shoulder Arthroplasty
Joaquin Sanchez-Sotelo*
Article Information
Identifiers and Pagination:
Year: 2011Volume: 5
First Page: 106
Last Page: 114
Publisher ID: TOORTHJ-5-106
DOI: 10.2174/1874325001105010106
Article History:
Received Date: 9/10/2009Revision Received Date: 10/4/2010
Acceptance Date: 10/7/2010
Electronic publication date: 16/3/2011
Collection year: 2011

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Shoulder arthroplasty has been the subject of marked advances over the last few years. Modern implants provide a wide range of options, including resurfacing of the humeral head, anatomic hemiarthroplasty, total shoulder arthroplasty, reverse shoulder arthroplasty and trauma-specific implants for fractures and nonunions. Most humeral components achieve successful long-term fixation without bone cement. Cemented all-polyethylene glenoid components remain the standard for anatomic total shoulder arthroplasty. The results of shoulder arthroplasty vary depending on the underlying diagnosis, the condition of the soft-tissues, and the type of reconstruction. Total shoulder arthroplasty seems to provide the best outcome for patients with osteoarthritis and inflammatory arthropathy. The outcome of hemiarthroplasty for proximal humerus fractures is somewhat unpredictable, though it seems to have improved with the use of fracture-specific designs, more attention to tuberosity repair, and the selective use of reverse arthroplasty, as well as a shift in indications towards internal fixation. Reverse shoulder arthroplasty has become extremely popular for patients with cuff-tear arthropathy, and its indications have been expanded to the field of revision surgery. Overall, shoulder arthroplasty is a very successful procedure with predictable pain relief and substantial improvements in motion and function.
INTRODUCTION
Over the last few years, shoulder arthroplasty has undergone substantial improvements. Detailed anatomic studies about the morphology of the proximal humerus have facilitated the design of newer modular implant systems that allow a more anatomic humeral head replacement. In patients with osteoarthritis or inflammatory arthropathies and glenoid disease, total shoulder arthroplasty seems to be superior to hemiarthroplasty. The risk of glenoid loosening seems to have decreased with improvements in implant design and surgical technique. For complex proximal humerus fractures, outcomes of hemiarthroplasty have improved due to better patient selection, the development of fracture-specific implants, careful tuberosity reconstruction, and shoulder immobilization for the first few weeks after surgery. Reverse shoulder arthroplasty has emerged as a very attractive alternative for patients with cuff-tear arthropathy, and its indications continue to expand, especially for revision surgery. Complications after shoulder arthroplasty may include infection, instability, neurovascular injury, stiffness, cuff tear, periprosthetic fractures, glenoid erosion and component loosening. The results of revision surgery have continued to improve over time.
THE HUMERAL COMPONENT: FIXATION AND DESIGN
Fixation
The fixation and design of the humeral component for anatomic shoulder arthroplasty has improved substantially over time. The component initially designed by Neer was a monoblock component with a smooth surface. It was initially fixed with polymethylmethacrilate; however, surgeons started to implant this component without cement in patients with good bone quality [1, 2]. Later studies demonstrated that for this particular component cementless fixation was associated with a high incidence (over 50%) of progressive radiolucent lines and/or migration [3, 4], not surprising taking into account that successful cementless fixation usually requires some texturing of the component surface.
Cemented fixation of the humeral component provides several advantages: it is associated with a very low rate of mechanical failure, antibiotics may be added to the cement to prevent infection, and it allows adequate positioning of the humeral component in patients with poor bone quality, proximal humerus fractures, or deformity. However, cementless fixation is very attractive, especially due to the difficulties associated with extracting well-fixed cement from the humeral canal in revision surgery. There are also isolated case reports of iatrogenic radial nerve injuries secondary to cement extrusion through the nutrient artery foramen. Cement pressurization is generally not recommended, and a bone plug should be used to limit distal migration of the cement.
Most modern humeral components are manufactured with surface texturing that has been associated with improved fixation compared to Neer smooth component [5]. There are only a few studies regarding the outcome of cementless fixation in shoulder arthroplasty. Sperling et al. reported a 6% radiographic mechanical failure rate with the first generation of the Cofield component, which was porous coated only underneath the humeral head. Most current components are manufactured with ingrowth surfaces that extend a variable amount below the humeral head level and provide adequate mid to ling-term fixation [6]. For primary surgery, it is probably best to select a component that does not achieve fixation through the whole length of the stem in order to facilitate later revision surgery.
We currently favor cementless fixation of the humeral component for most patients (Fig. 1), and limit cement fixation when it is absolutely required, such as in proximal humerus fractures or when adequate primary stability of the humeral component cannot be achieved even with proximal humerus cancellous bone grafting [7].
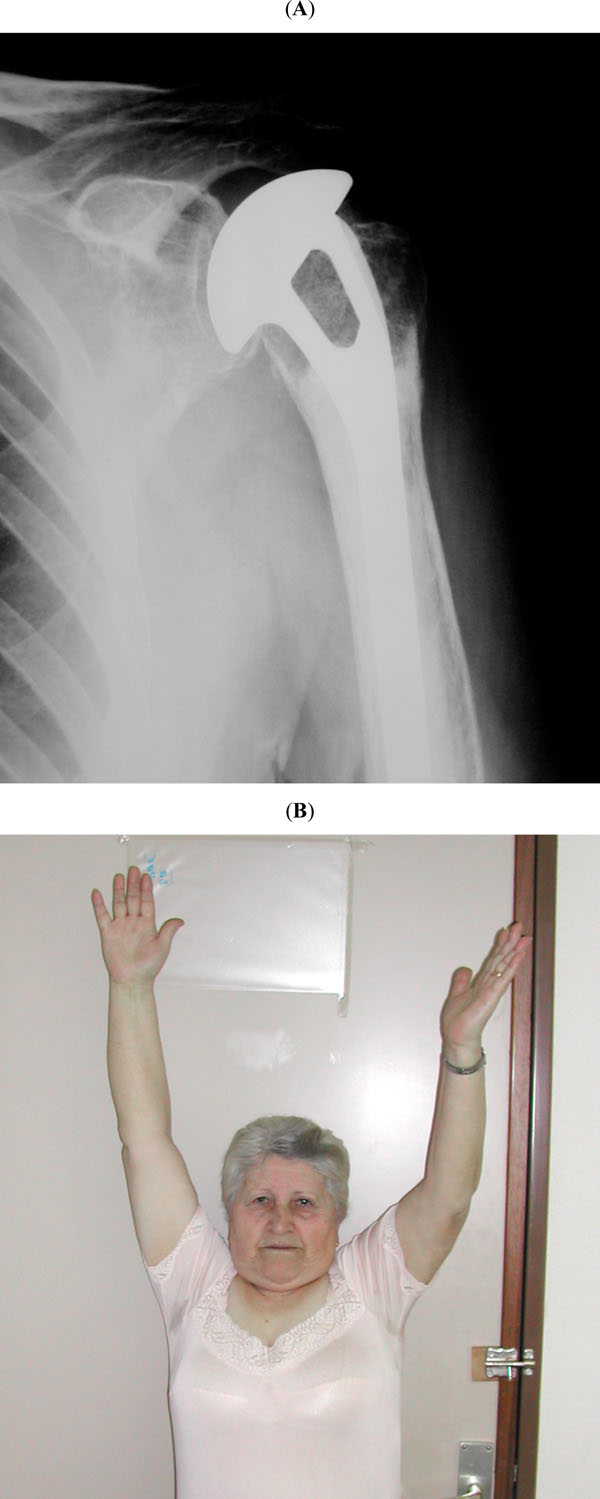
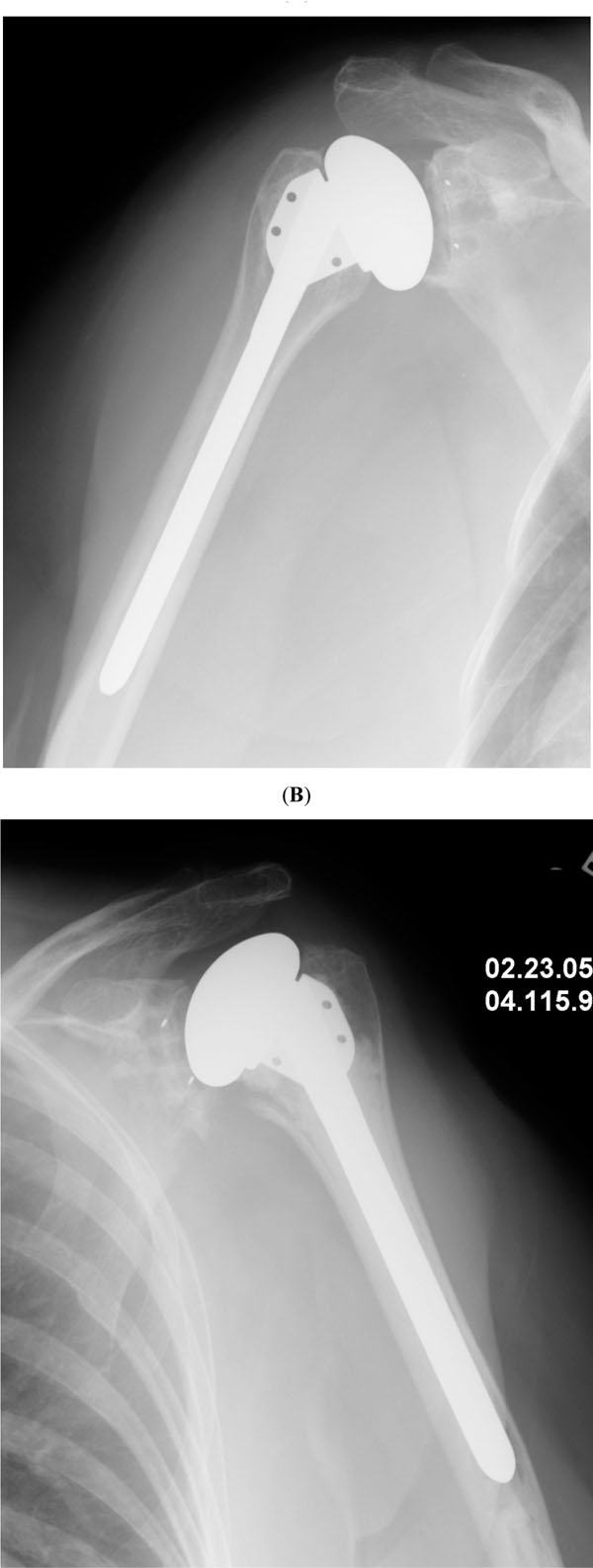 |
Fig. (1). Anteroposterior radiographs corresponding to two examples of shoulder arthroplasty using cementless (A) and cemented (B) humeral components. |
Design
There have been three major generations of anatomic humeral components based on their design. First generation components were monoblocks manufactured in a very limited number of sizes. Second generation components were characterized by the introduction of modular heads and ingrowth coating. Modular heads facilitate selection of the ideal head size for each particular patient in order to balance the soft tissues. They also facilitate revision surgery by allowing removal from the stem. Interestingly, it has been difficult to demonstrate improved clinical outcomes by using modular components [8].
The design of third generation components was prompted by several anatomic studies analyzing the relative variability of some anatomic parameters [9, 10]. These third generation humeral components are commonly referred to as anatomic or adaptable [11]. Depending on the design used, these components allow adjustments of the prosthetic humeral head position referenced to the stem in the anteroposterior and mediolateral directions. Some implants also allow for various degrees of head inclination. It is important to understand that the terminology used for different systems may change. For example, in the Aequalis system the term offset refers to the relationship between the center of the humeral head and the center of the humeral stem; in the Cofield system, such distance is referred to as eccentricity, whereas offset is used to name prosthetic heads with extra thickness for a given diameter, which would lateralize the humerus compared to the glenoid.
Another interesting option for selected patients is the use of so-called resurfacing implants [12, 13]. Some authors have published a high rate of satisfactory outcomes using resurfacing implants fixed with cement or coated with hydroxyapatite. These components do not have an intramedullary stem, and to a given extent they reproduce anatomically the morphology of the proximal humerus as the cover the native humeral head. They are especially attractive in patients with associated proximal humeral deformity that would otherwise require an associated osteotomy or stem bending. They may also be useful in rheumatoid patients requiring elbow arthroplasty. The stem of the humeral component of the elbow prosthesis may interfere with insertion of the shoulder component or leave a very narrow segment between the two stems, which may increase the risk of a periprosthetic fracture. An additional advantage of resurfacing arthroplasty is an easier revision surgery on the humeral side if it became necessary.
Resurfacing arthroplasty has some disadvantages as well: it may be difficult to achieve adequate stability of the prosthesis if the local bone stock is compromised. In addition, the implantation of a glenoid component is more difficult, as the exposure is limited by preservation of the humeral head. Soft-tissue balance may also be difficult to achieve, as the size and position of humeral head cannot be altered much. Finally, it is difficult to assess the radiographic bone-implant interface.
THE GLENOID COMPONENT: FIXATION, DESIGN AND INDICATIONS
Fixation and Design
Most glenoid components are fixed with polymethylmethacrilate. The traditional component is an all-polyethylene implant with a slightly convex backside and a keel to be inserted into the glenoid vault. Most currently used components have their keel replaced by two or more pegs. There are multiple peg configurations. Some components are designed for hybrid fixation, where one of the pegs is designed for ingrowth and fabricated as either a grooved all-polyethylene peg or a metal ingrowth peg. There is interest in so-called self-pressurizing glenoids, with pegs designed to provide and maintain pressure while the cement is curing. Pegged components seem to allow more accurate preparation of the glenoid bone and have been associated in some studies to a lower rate of radiolucent lines in the immediate postoperative radiograph [14]. However, in patients with bone loss it may not be possible to implant a pegged component, and a keeled component may be required (Fig. 2).
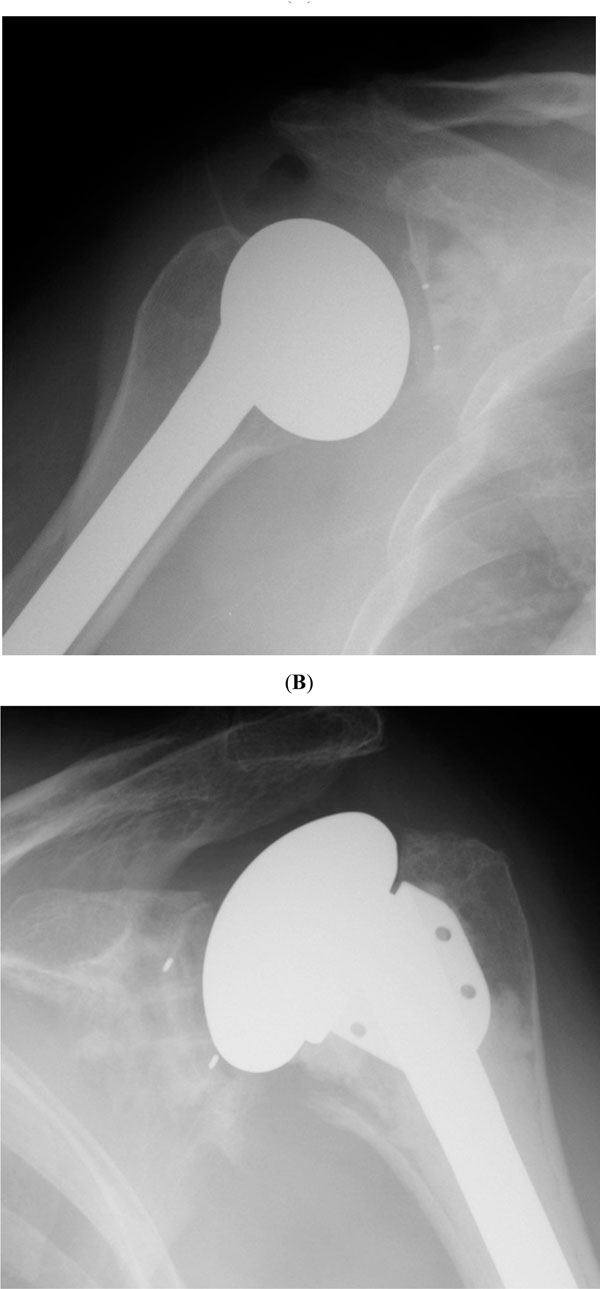 |
Fig. (2). Radiographs corresponding to cemented pegged (A) and keeled (B) glenoid components. Note the quality of the cementing technique with absence of radiolucent lines. |
Despite improvements in design and cementing techniques, glenoid loosening continues to be a relatively common mode of failure after total shoulder arthroplasty [15, 6]. Cementless fixation remains an attractive alternative, but unfortunately the first generation of uncemented implants were associated with a high failure rate [16-18]. These components had relatively thin polyethylene inserts in order to avoid overstuffing of the glenohumeral joint, and they failed secondary to catastrophic polyethylene wear or dissociation. New materials such as porous titanium or tantalum may allow manufacturing of more successful cementless glenoid components in the future.
There is some controversy regarding the ideal relationship between the radius of curvature of the prosthetic humeral head and the articular surface of the glenoid component. In general, most surgeons favor a few millimeters of mistmatch, which creates a less constrained joint and may be associated with less radiolucent lines [19].
Indications
Controversy persists about the indications for implantation of a glenoid component as opposed to a hemiarthroplasty. Some state that total shoulder arthroplasty and hemiarthroplasty provide similar rates of pain relief and satisfactory outcome, and the risk of late glenoid component loosening cannot be neglected. There are other reasons that are not commonly mentioned but probably influence the decision to perform a hemiarthroplasty and not a total shoulder arthroplasty: glenoid exposure is difficult, glenoid implantation requires more assistance and prolongs surgical time, and the difference in reimbursement is not large. However, most current literature clearly shows that for patients with osteoarthritis and rheumatoid arthritis, total shoulder arthroplasty is more reliable in terms of both pain relief and functional improvements [20, 21]. Interestingly, revision of a failed hemiarthroplasty secondary to progressive glenoid erosion seems to be more common than revision of a failed total shoulder arthroplasty for glenoid loosening [22].
The main risk factors for glenoid component loosening include poor remaining bone stock, rotator cuff deficiency leading to superior humeral migration (eccentric contact may facilitate glenoid loosening [23]), and a poor cementing technique. In patients with a completely normal glenoid articular cartilage (early osteonecrosis, proximal humerus fractures) implantation of a glenoid component is unnecessary. Table 1 summarizes the relative indications of hemiarthroplasty and total shoulder arthroplasty.
Indications for Hemiarthroplasty and Total Shoulder Arthroplasty
| Hemiarthroplasty | Osteonecrosis with a preserved glenoid Proximal humerus fractures Cuff-tear arthropathy Inflammatory arthropathy (rheumatoid) if
|
| Total shoulder arthroplasty | Primary osteoarthritis Post-traumatic osteoarthritis Inflammatory arthropathies Osteonecrosis with an affected glenoid |
RESULTS OF SHOULDER ARTHROPLASTY
The results of shoulder arthroplasty vary depending on the underlying diagnosis, the condition of the joint and the soft-tissues at the time of surgery, and the type of reconstruction performed. The results of shoulder arthroplasty in primary osteoarthritis are satisfactory in a large number of patients: pain is improved in over 90% of the individuals, and their average elevation is usually over 135 degrees. For this particular diagnosis, total shoulder arthroplasty seems to be superior to hemiarthroplasty [24]. This was clearly shown in a prospective randomized study by Garstman et al. [25] and a long-term 15 year study by Sperling et al. [22].
The results of shoulder arthroplasty in osteonecrosis are similar to osteoarthritis. Hattrup and Cofield reported in 88 patients with osteonecrosis who received a hemiarthroplasty or a total shoulder arthroplasty [2]. Pain and satisfaction improved in 80% of the patients. The results in posttraumatic osteonecrosis and other sequels of proximal humerus fractures are less satisfactory [26, 27] Worse results are to be expected when tuberosity osteotomies are required to correct for the underlying deformity.
Shoulder arthroplasty provides satisfactory results in a large number of patients with inflammatory arthritis as well. Sperling et al. reported on 303 consecutive shoulder arthroplasties performed at the Mayo Clinic for inflammatory arthritis [21]. Both hemiarthroplasty and total shoulder arthroplasty provided significant improvements in pain relief and function. Total shoulder arthroplasty was superior to hemiarthroplasty in patients with an intact rotator cuff; it was equivalent to hemiarthroplasty when the cuff was thin or torn. Other authors have reported similar results [28].
The results of hemiarthroplasty in cuff-tear arthropathy are suboptimal. Sanchez-Sotelo et al. reported on 33 consecutive hemiarthroplasties for cuff-tear arthropathy [29]. Pain improved in approximately 70% of the shoulders, but mean active elevation was only 90 degrees (Fig. 3). As mentioned below, reverse shoulder arthroplasty is now felt by most to be the procedure of choice for patients with cuff-tear arthropathy.
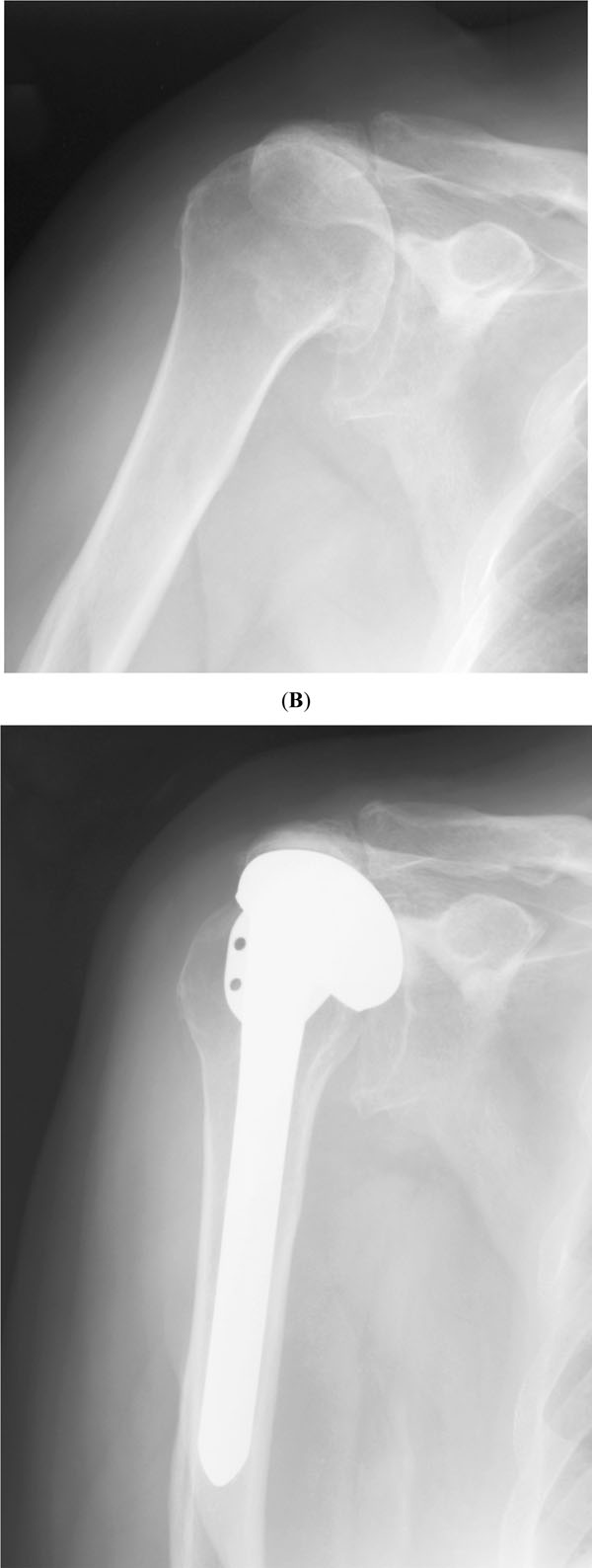 |
Fig. (3). Radiographs taken before surgery (A) and two years (B) after shoulder hemiarthroplasty for cuff tear arthropathy. |
SHOULDER ARTHROPLASTY IN FRACTURES
Indications
The indications for shoulder hemiarthroplasty in fractures has been refined over the last few years due to better understanding of the different fracture patterns, the common use of CT scan for fracture imaging, and improvements in internal fixation devices and techniques. Shoulder hemiarthroplasty is still considered for classic four part proximal humerus fractures, head-impaction fracture and head splitting fractures. Valgus-impacted four part fractures and three-part fractures in the presence of osteopenia were considered in the past indications for shoulder hemiarthroplasty, but are now more commonly treated with internal fixation.
Principles
Shoulder hemiarthroplasty for proximal humerus fractures is a technically demanding procedure. Most anatomic references used to position the humeral component in shoulder arthroplasty are lost in displaced fractures. In addition, sound tuberosity reconstruction is paramount for the success of this procedure, and it is difficult to achieve. A few general principles are useful in order to obtain a satisfactory outcome:
- The implant selected must facilitate tuberosity reconstruction. Some implants have a very bulky proximal body, which may interfere with tuberosity reduction and healing. Many current systems have dedicated fracture stems with a narrow proximal section and porous or hydroxiapatite coating (Fig. 4).
- Strong tuberosity fixation is required. Most surgeons use a combination of horizontal and vertical heavy non-absorbable sutures through the implant, rotator cuff insertion, and the diaphysis.
- The overall morphology of the proximal humerus needs to be recreated. Most common mistakes include cementing the humeral component too proud or too low and lack of restoration of an adequate head-tuberosity relationship.
- Bone autograft from the fractured humeral head should be placed between the tuberosities and the diaphysis, avoiding cement interposition between the fragments.
- The shoulder should be immobilized for a few weeks in some internal rotation; marked internal rotation may place excessive stress on the greater tuberosity.
- Tuberosity healing and stability are more important than early motion. Currently, most surgeons favor shoulder immobilization for 4-6 weeks after surgery.
Outcome
Published results about the outcome of shoulder hemiarthroplasty for proximal humerus fractures have varied tremendously. Neer published a large rate of satisfactory results in his initial study [30]. Most studies published later have reported a higher rate of failure. In the absence of complications, most patients experience little pain, but their motion and strength varies depending on the quality of the reconstruction and tuberosity healing. Average elevation is approximately 90 to 100 degrees, although it may range less than 30 to more than 150 degrees (Fig. 5). The Mayo Clinic experience with shoulder arthroplasty for acute proximal humerus fractures was recently reported in a group of 57 patients followed for a minimum of 5 years [31]. Overall results were considered unsatisfactory in 50% of the shoulders, and their mean active elevation and active external rotation were 100 and 30 degrees respectively. For these reasons, there is some interest in the use of reverse shoulder arthroplasty for acute proximal humerus fractures.
REVERSE SHOULDER ARTHROPLASTY
Rationale
Reverse shoulder arthroplasty has emerged as a very popular alternative for replacement surgery in the absence of a functional rotator cuff. The term “reverse” refers to the shape of the articulating components: the glenoid component has a convex spherical articular surface, and the articulating portion of the humeral component is a concave polyethylene insert (Fig. 6) [32]. Patients regain active elevation due to the semiconstrained nature of the implant and an improved deltoid lever arm [33]. Other constrained designs used in the past were associated with a high rate of mechanical failure. Reverse arthroplasty has been associated with an acceptable failure rate so far, partly because of better primary stability of the glenoid component and partly due to the medial location of the shoulder center of rotation in reference to the glenoid baseplate.
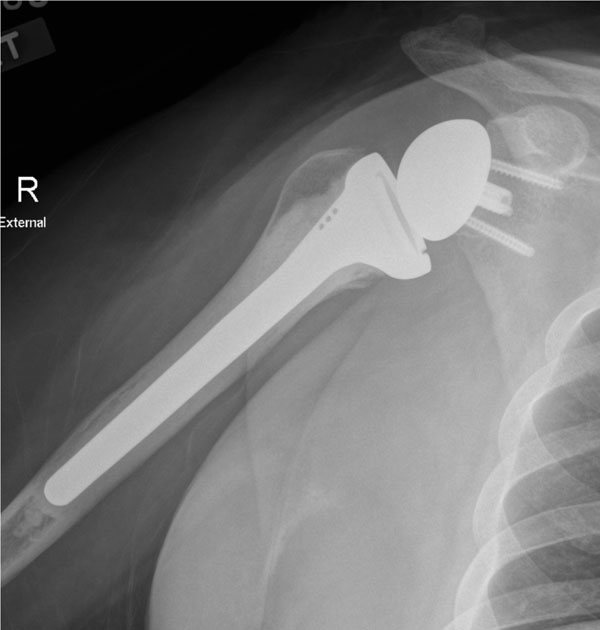 |
Fig. (6). Anteroposterior radiograph after implantation of a reverse prosthesis. |
Indications
The main indication for reverse shoulder arthroplasty is cuff-tear arthropathy. Reverse shoulder arthroplasty is very successful in this condition, with substantial improvements in pain and active elevation [34]. The indications for reverse shoulder arthroplasty have been expanded to include selected proximal humerus fractures, nonunions and malunions, as well as revision surgery in the presence of gross instability or absence of rotator cuff or tuberosities, reconstruction after resection of tumors, and massive cuff tears with pseudoparalysis but no arthritis [32, 35] (Table 2).
Indications for Reverse Shoulder Arthroplasty
|
|
|
|
|
|
|
|
Outcomes
The first large outcome study reporting the results of Dr. Grammont’s reverse prosthesis was published by Sirveaux et al. in 2004 [36]. These authors reported on 80 patients with a mean follow-up of 3.6 years. The procedure was associated with good pain relief in 96% of the patients, mean active elevation increased from 73 to 138 degrees, and Constant scores improved from 22 to 65 points. There were five cases of aseptic loosening and seven of glenoid dissociation, and three implants were revised. The integrity of teres minor was found to be essential for recovery of external rotation and was associated with better Constant scores. Slightly worse results were reported by Werner et al. [37], also using the Delta III implant.
The longest follow-up study to date was reported by Guery et al. [38]. These authors reported on 80 prostheses implanted mostly in patients with cuff-tear arthropathy. The 10-year survivals free of revision surgery and glenoid loosening were 91% and 84%; however, there was a functional deterioration over time, with less than 60% survivorship of an absolute Constant score over 30 points. These authors hypothesized occult aseptic glenoid loosening as a possible explanation for the functional deterioration over time.
Several authors have noted the influence of the underlying diagnosis on the expected outcome. Boileau et al. [39], reported on 45 reverse arthroplasties performed for cuff-tear arthropathy (21), sequelae of fracture (5) and revision shoulder arthroplasty (19). The complication rate was 24%, including dislocation (3), deep infection (3), humeral aseptic loosening (1), periprosthetic fractures (3), late acromial fractures (2), and axillary nerve palsy (1). There were better results and fewer complications in the group of patients with cuff-tear arthropathy. Similar findings were reported by Wall et al. [35] in a larger series of 240 reverse shoulder arthroplasties, worse results were reported when reverse arthroplasty was used for revision surgery.
Dr. Frankle’s group has reported on the results obtained using a reverse prosthesis with a lateral center of rotation. In 2005, these authors reported a minimum two-year follow-up study of sixty shoulders with cuff-tear arthropathy [40]. Reverse arthroplasty was associated with statistically significant improvements in pain and function, with a mean active elevation of approximately 105 degrees. However, there was a 17 % complication rate and a 12% rate of revision for implant failure. Some features of the implant were modified to improve screw fixation of the glenoid component, and the results of the new version of this implant has been reported recently. Cuff et al. [34], reported on 96 shoulders followed for a minimum of 2 years with similar improvements in pain and function, but no cases of either mechanical failure of the glenoid or notching.
COMPLICATIONS AND REVISION SURGERY AFTER SHOULDER ARTHROPLASTY
Infection
Infection rates after shoulder arthroplasty are relatively low. However, a relatively large percentage of periprosthetic deep infections after shoulder arthroplasty are difficult to identify preoperatively, as a large number of patients with intraoperative positive cultures do not show abnormalities in their preoperative studies (white cell blood count, sedimentation rate, C reactive protein, bone scan, aspiration) [41]. This may be due to the high prevalence of slow growing microorganisms such as Propionibacterium acnes. Two-stage reimplantation is the treatment of choice for most deep infections after shoulder arthroplasty. The rate of reinfection after reimplantation is low, but the functional results are oftentimes compromised by stiffness and cuff dysfunction [42, 43].
Instability
The rate of instability after shoulder arthroplasty is estimated to be 5%. Most instability cases are secondary to a combination of poor component position and soft-tissue imbalance [44]. Anterior instability is associated usually with subscapularis deficiency. Primary tendon repair is associated with a high rate of failure, and allograft augmentation or pectoralis major transfer may be required. Posterior instability usually requires component revision, lengthening of the anterior soft tissue structures, and tensioning of the posterior capsule and rotator cuff. We reviewed the Mayo Clinic experience with revision surgery for prosthetic instability, and found about a 60% rate of persistent instability after reoperation [44]. Currently, most surgeons favor revising the unstable shoulder arthroplasty to a reverse arthroplasty, but there is not any published report on this particular topic.
Periprosthetic Fractures
Humeral periprosthetic fractures may happen intraoperatively or after surgery. Intraoperative fractures are especially common in rheumatoid arthritis, and usually happen when the humerus is rotated for exposure. Prevention is key, and may be accomplished by careful and extensive soft-tissue releases before applying any torsion to the humeral shaft, as well as the selective use of the so-called anteromedial approach [45]. Humeral shaft periprosthetic fractures may be treated non-surgically if the implant is well fixed and the fracture line is located at or distal to the tip of the prosthesis. Otherwise, surgery is required and may involve internal fixation with cerclage, plates and/or bone struts with or without revision of the humeral component [46].
Glenoid Complications
As noted before, the glenoid side of the shoulder joint seems to be much more problematic than the humeral side. On one hand, patients with glenoid arthritis receiving a hemiarthroplasty may continue to experience pain and require revision surgery for placement of a glenoid component [47]. On the other hand, there is a relatively high rate of concerning radiolucent lines around cemented glenoid components. With modern cementing techniques, the rate of glenoid radiographic loosening is probably around 15% [48]. Failed glenoid components may be revised to a new component provided there is enough remaining glenoid bone stock. Otherwise, the total shoulder arthroplasty needs to be revised to a hemiarthroplasty with bone grafting of the glenoid. Pain relief seems to be better when another glenoid component can be implanted [49].
Other
Shoulder arthroplasty may be complicated by brachial plexopathy, stiffness, rotator cuff tearing, heterotopic ossification and others. Most brachial plexopathies represent transient neuroapraxias that recover over time [50].
SUMMARY
Shoulder arthroplasty has improved tremendously over the last two decades. Currently, a large array of implants is available for shoulder reconstruction in different conditions. Anatomic total shoulder arthroplasty is the treatment of choice for most patients with primary and posttraumatic osteoarthritis as well as inflammatory conditions of the shoulder. Hemiarthroplasty may need to be considered for patients with no glenoid involvement or severe rotator cuff deficiency. The outcome of hemiarthroplasty for acute proximal humerus fractures or the sequels of trauma seems to be less predictable. Reverse shoulder arthroplasty has emerged as the treatment of choice for patients with cuff-tear arthropathy, and its indications are being expanded to revision surgery and selected proximal humerus fractures and nonunions. Overall, shoulder arthroplasty provides good pain relief to most patients undergoing this procedure; however, these procedures are not devoid of complications, sometimes requiring revision surgery.