All published articles of this journal are available on ScienceDirect.
The Role of Different Hyaluronic Acids in the Articular Cartilage of Rabbit
Abstract
Purpose:
To elucidate if the differences found in the physico-chemical and rheological behaviour of Hyaluronic Acids result in different in vivo activity. For this purpose two Hyaluronic Acids (HA), HA-1 and HA-2, with similar molecular weight but different percentage of concentration variation, were compared through an osteoarthritis model.
Methods and Materials:
Osteoarthritis was induced in white New Zealand rabbits by anterior cruciate ligament section. After the induction period, the animals were allocated to receive HA-1 or HA-2 intra-articularly in one knee whereas the contralateral knee was used as Operated Control. An additional group of non-operated animals was used as Healthy Controls. Samples of cartilage were taken for different measures: apoptosis, nitric oxide (nitrites) and hyaluronic acid in synovial fluid.
Results:
The administration of HA-1 had a significant inhibitor effect on apoptosis of the chondrocytes compared to operated untreated animals (p = 0.0089), whereas this difference was not observed in the HA-2 knees. Levels of nitrites determined by HPLC in the HA-1 knees were similar to those in the Healthy group (p = 0.6551) whereas they were significantly higher in Operated Control and HA-2 groups (p = 0.0001). The comparison between HA-1 and HA-2 also revealed significantly lower levels of nitrites in the HA-1 knees (p = 0.0001). Values of hyaluronic acid in synovial fluid did not show statistical differences between the different study groups.
Conclusions:
HA-1 and HA-2 showed different physico-chemical characteristics and these differences have resulted in different in vivo behaviour. As a consequence, not all the HA with similar molecular weight can be considered as equivalent.
INTRODUCTION
Hyaluronic Acid (HA) is responsible for the viscoelastic properties of synovial fluid, which is greatly diminished in osteoarthritic joints in both concentration and molecular weight [1]. The main objective of intra-articular treatment with HA is to restore the viscoelastic properties of the synovial fluid. In a recent review of existing treatment guidelines, HA is recommended for the treatment of knee OA (level of evidence Ia) [2]. It has been demonstrated that HA reduces osteoarthritic pain owing to a reduction of nociceptive and sensorial response in the articular pain receptors [3]. In the same way, in vitro studies [4, 5] have demonstrated the protective effects of HA on the cartilage in experimental models of osteoarthritis. Other in vitro studies have also demonstrated that HA has beneficial effects on the extra-cellular matrix [6], the immune system cells [7] and inflammation mediators [8]. There are several preparations of HA authorized for the treatment of osteoarthritis from different sources, manufacturing process, molecular weight and final concentration. A recent study [9] made it clear that the rheological properties, the molecular weight of HA and its concentration are factors which could determine the intra-articular viability of the molecule. Mechanical and chemical degradation were both reduced in HAs with low molecular weight, with the final concentration of the product being a critical factor. Considering the differences found, and to elucidate whether the different physico-chemical behaviour had, as a consequence, different in vivo activity, we decided to conduct the present study. The comparison is made using an experimental model of osteoarthritis developed by our team [10].
MATERIALS AND METHODS
Experimental Model
Osteoarthritis was induced in white New Zealand rabbits kept at the animal facilities of University of Leon, Spain. The study was performed in accordance with the laws in force relative to the protection of animals used for experimentation and other scientific purposes and was favourably informed by the Ethics Commission of the University of León. Postoperatively, the rabbits were housed in a cage, after which they were allowed unlimited activity. During follow-up no joint immobilization was used.
Hyaluronic Acid
Two Hyaluronic Acids were studied, both have similar source (biofermentation), mechanical and optical properties, but one revealed fluctuating concentrations in a prior study (Prieto JG et al. HA-2 has a 15% concentration variation (8.39 ± 1.65 mgml-1) while other HA studied (including HA-1) have a 7% variation. HA-1 (900 KDa) (AdantÒ Tedec-Meiji Farma, Spain) and HA-2 (1200 KDa) (OstenilÒ TRB Chemedica AG, Germany). Depolimerisation processes is highly dependent on the sample concentration, HA-2 results in higher depolymerisation than HA-1 with tetracyclines and thermal depolymerisation [9].
Osteoarthritis Induction
Anterior Cruciate Ligament (ACL) section was performed, based on the Pond-Nuki model [11], under local anaesthesia by percutaneous surgery through a 2-3 mm incision using a curved scalpel (Cutfix Stitch Cutter, B. Braun Surgical GMBH) [10].
Study Design
In total 36 animals were used and the ACL of both knees were sectioned in all of them. After ten weeks’ development of osteoarthritis, the animals were randomly assigned 1:1 to receive HA1 or HA2. HA was injected intra-articularly into one knee for three consecutive weeks at a dose of 0.1 ml/kg weight (0.3 ml/knee, in rabbits with an average weight of 3kg) one injection each week, leaving the other knee to be used as Operated Control. The animals were sacrificed two weeks after the last HA injection was administered. Control Group: Four untreated animals of the same age as the operated animals, not having undergone surgery were used as Healthy Control (8 control knees).
After a 500µl saline injection, samples of synovial fluid were taken from all knees, where possible, so as to quantify the HA molecular weight and concentration by High Performance Liquid Chromatography (HPLC) [9, 10, 12-14]. Gross morphological changes to the femoral condyles, trochlea and tibial surfaces in the rabbits were assessed during the collection of cartilage samples according to the method and the grading scale reported by Yoshiaka et al. [15]. The entire surface of both femoral condyles (internal and external to obtain a greater number of chondrocytes) was taken in order to measure apoptosis by TUNEL and cytometry. TUNEL method as a Gold Standard method [16-19] was done after initial fixation of the samples with 10% formaldehyde, paraffin embedded cut into sections which were processed (ApopTag® Plus peroxidase in situ Apoptosis Detection Kit. Chemicon International, USA), cell in apoptosis were quantified by an external department and evaluated blindy. Cytometry with Annexin Kit (BD Pharmingen, San Jose, CA USA), after 6 hours cartilage hyaluronidase (Sigma H-3884; 0.1mg/ml) and IIS-type collagenase (C-1764; 2mg/ml) digestion [20-22]. Cartilage from the tibial plateau was used for the indirect quantification of nitric oxide in cultured cartilage using the Griess method. The supernatant of cartilage digestion for cytometry study was used for nitrites and nitrates studies by HPLC [23, 24].
Statistical Analysis of Data
The Mann-Whitney Test was used to analyse the results of gross morphology. All the remaining parameters studied were analysed using ANOVA–MANOVA with the Newman-Keuls Test. Statistical significance was set at p < 0.05.
RESULTS
Gross Morphology (Fig. 1)
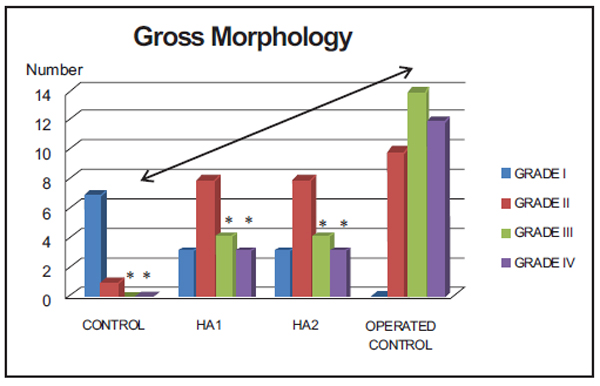
*Grade III and IV: Operated Control vs control (p = 0.002), Operated Control vs treated groups (p = 0.003).
When considering the most severe grades (III and IV) altogether, statistically significant differences were found between Operated Contol group and both Control (p = 0.002) and Treated groups (HA1 or HA2) (p = 0.003). No differences were found between Control and Treated groups (p = 0.185).
Levels of Apoptosis and Necrosis (Cytometry) (%) (Fig. 2).
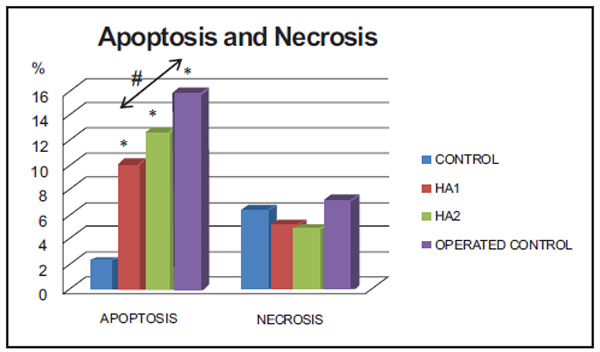
*Operated Control, HA2 and HA1 groups versus Control (p = 0.0018, 0.0001, 0.0001), # HA1 versus Operated Control group (p = 0.0089).
Apoptosis grade. TUNEL method (%) (Fig. 3).

No statistically significant differences were found in any of the comparisons made between groups.
Nitrites and nitrates in supernatant (µg/ml) (HPLC) (Fig. 4).

*Operated Control and Ha2 versus Control (p = 0.0001, 0.0001), ♦ Operated Control versus Ha2 (p = 0.0248), # HA1 versus Operated Control and HA2 (p = 0.0001, 0.0001), HA1 versus control (p = 0.5245).
Hyaluronic Acid in Synovial Fluid (HPLC) (Fig. 5).
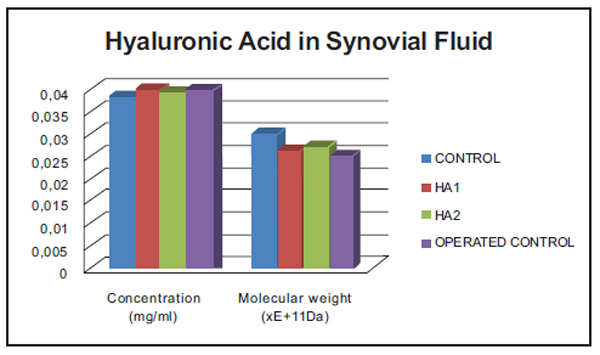
No statistically significant differences were found in any of the comparisons made between groups.
DISCUSSION
The administration of HA1 for three weeks in rabbits after ten weeks osteoarthritis-induction, had an inhibiting effect on the apoptosis of the chondrocytes compared to the operated Control group (cytometry study), with statistically significant differences (p = 0.0089) similar to that observed in a five week study [10]. However, this difference was not reached in the group treated with HA2 (p = 0.0696). No differences between either treatment groups was found (p = 0.2176). The study of necrosis did not reveal statistically significant differences between the different study groups. The apoptosis levels determined by TUNEL were clearly higher in the Operated Control group, although the high standard deviation values made the differences not statistically significant. This method has been, up to now, considered the Gold Standard [16, 25, 26]. Nevertheless its variability has been questioned in recent investigations. A new method to replace the TUNEL method for another with better possibilities of quantification (for instance chondropoptosis determined by caspase-2L or caspase-3) must therefore be considered for future studies [27]. Gross morphological study did not revealed differences, the advantage of this method regarding a complete visual histological assessment scale (ICRS) [28], is that in small experimental animals as rabbits, valuable samples are not lost they can be employed in other interesting study methods as flow cytometry. In the near future probably immunohistochemical staining should further displace conventional histological techniques [28].
Levels of nitrites in the knees of both groups treated were significantly lower than those in the Operated Control group. Nevertheless, levels in the animals treated with HA1 were very similar to those obtained in the Control group (p = 0.6551), whereas those treated with HA2 were not (p = 0.0001). This was confirmed by the comparison between HA1 and HA2 that revealed significantly lower levels of nitrites in the HA1 group (p = 0.0001). Levels of nitrates were five to eight times lower than nitrites and follow the same pattern of distribution in the different groups; nevertheless, there were no statistically significant differences, probably due to the high standard deviation values found. The differences found when analysing nitrites are maintained when both nitrites and nitrates are considered together as stable metabolites of nitric oxide. Values of HA in synovial fluid did not show any statistical differences between the different study groups. The molecular weight of the HA in the synovial fluid of the operated Control knees was 17% lower than in the control group. The groups treated showed less reduction of HA molecular weight (10% HA2, 13% HA1) as could be expected after the administration of exogenous HA and in accordance with the molecular weights of HA1 and HA2, respectively. According to a consensus reached at the 7th International Conference on hyaluronan (South California, 2007) both HAs are included as HAs of medium molecular weight, and both have potentially disease-modifying effects [29] as inhibition IL-1β stimulated production of MMP-1, MMP-3 and MMP-13 [30].
Considering the results shown above, it seems to be clear that in vivo activity is not influenced by molecular weight. Recent studies published by our group [9] stated that considering the elasto-viscous nature of HA, not only molecular weight but stability against degradation processes by mechanical and chemical factors, is an important factor. Rheological properties and final concentration are also critical aspects to be taken into account in the final behaviour of the molecule when injected into the articular space. In the study, differences were also found among the HAs of similar molecular weight, especially with HA2 where the fluctuating concentrations observed led to lower stability results than expected [9]. In this study, the animals treated with HA1 not only showed statistically significant lower NO levels regarding untreated animals, but the values were also similar to the ones in the healthy Control group. The animals treated with HA2 showed lower NO values than the untreated animals, but levels were much higher than the control group (statistically significant). In addition the comparison between treatment groups, HA1 and HA2, revealed significantly lower levels of nitrites in the HA1 group. It is known that NO is able to induce apoptosis in chondrocytes and that, moreover, osteoarthritic cartilage contains a higher percentage of cells undergoing apoptosis than normal cartilage [31]. In conclusion, the protective role of HA1 on the osteoarthritic cartilage is also confirmed with similar results to those obtained in previous studies [10]. HA1 and HA2 showed different physico-chemical characteristics and these differences have resulted in different in vivo behaviour. Consequently, the two products cannot be considered as equivalent. HA1 has shown higher effects on inflammation processes than HA2, which could probably provide better results and a long-term protective effect. Along the same line of research, a long-term clinical trial is under development to confirm this protective action in humans, and a new study focus on extracellular matrix is required to confirm differences founded in this study.
CONFLICT OF INTEREST STATEMENT
Part of this study has been supported by Tedec-Meiji Farma as a project integrated in the University-Industry collaboration program issued by the University of León (Spain). As an university project no economical benefits in any form have been received or will be received from a commercial party directly or indirectly to the subject of this article by the authors. Our group has been working in Hyaluronic Acid projects with this University-Industry plan with almost all Hyaluronic Acid companies including TRB Chemedica AG.
ACKNOWLEDGEMENTS
We would like to thank Claire Byrne for her contribution in the translation of the manuscript.
This study was supported by a grant from the University-Industry agreement plan.


