All published articles of this journal are available on ScienceDirect.
Pilot Study on a Far Infrared Ray Emitting Compression Knee Sleeve for Symptom Relief in Knee Osteoarthritis
Abstract
Introduction
This pilot study investigated the potential clinical benefits of a compression knee sleeve containing Far-Infrared Ray (FIR) emitting particles in patients with knee Osteoarthritis (OA).
Methods
We conducted a retrospective cohort study at a single institution. Among 48 patients initially fitted with FIR knee sleeves, 22 patients (30 sleeves) had complete one-month follow-up data and were included in the analysis. Two groups were identified: (1) patients who received only the FIR knee sleeve (HA-/Sleeve+), and (2) patients who received both the FIR sleeve and Hyaluronic Acid (HA) injection concurrently (HA+/Sleeve+). Patient-Reported Outcome Measures (PROMs) were collected at baseline and one-month follow-up, including the Knee Injury and Osteoarthritis Outcome Score, JR (KOOS JR), Numeric Pain Rating Scale (NPRS), and a Five-Point Likert Satisfaction Scale.
Results
At one month, both groups demonstrated improvement in knee health, pain, and satisfaction. KOOS JR scores improved by 12.27 points in the HA-/Sleeve+ group and 9.809 points in the HA+/Sleeve+ group. Pain scores decreased by 3 points and 2 points, respectively. Satisfaction scores increased by 1.416 points in the HA-/Sleeve+ group and 0.278 points in the HA+/Sleeve+ group. Improvements were greater in the HA-/Sleeve+ group across all measures.
Discussion
Limitations include a small sample size, an inability to ensure knee sleeve compliance, and the need for further research to explore potential synergies between knee compression, injections, and other treatments.
Conclusion
This preliminary study suggests that a FIR-emitting compression knee sleeve may improve symptoms in patients with knee OA. The greater improvements observed in patients who did not receive HA injections may warrant further investigation. Larger prospective, randomized studies are necessary to validate these findings and define optimal use cases for this emerging nonoperative therapy.
1. INTRODUCTION
Osteoarthritis (OA) is a debilitating and complex joint disease that affects over 240 million people worldwide [1]. The Osteoarthritis Research Society International defines OA as a “molecular derangement (abnormal joint tissue metabolism) followed by anatomic, and/or physiologic derangements (characterized by cartilage degradation, bone remodeling, osteophyte formation, joint inflammation, and loss of normal joint function), that can culminate in illness” [2]. A notable feature of osteoarthritis is hypoxia, which, through the activation of hypoxia-induced transcriptional pathways, may lead to cartilage lesions [3-5]. These changes can result in joint dysfunction, pain, stiffness, functional limitation, and loss of valued activities. [5]
The knee is the most frequently affected joint, and in the United States, the incidence of knee OA in adults aged ≥ 45 years ranges from 7% to 17% [6, 7]. Thus, efforts have been made to target the various contributors to illness. First-line treatment for symptomatic knee OA includes physical therapy, patient education, weight loss, Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), and knee bracing. If these options fail to provide relief, intra-articular corticosteroid, hyaluronic acid, and platelet-rich plasma might be considered. Finally, surgical interventions include osteotomy, unicompartmental knee arthroplasty, and total knee arthroplasty [8-10]. However, treatments, such as NSAIDs and injections, are accompanied by a wide spectrum of risks [11]. NSAIDs are associated with heart disease, kidney injury, and gastrointestinal toxicity [12-14]. Salis and Sainsbury suggest that long-term NSAIDs use can accelerate the progression to total knee arthroplasty by markedly exacerbating symptoms [15]. Cortisone injections can also lead to joint-related side effects, including accelerated OA progression, subchondral insufficiency fractures, osteonecrosis, and rapid joint destruction [16]. In addition to risks, these treatments can also be expensive, as the average cost of nonoperative procedures per patient was $1,355 ± $2,087 in the year prior to total knee arthroplasty [17].
In recent years, bioactive compression knee sleeves have become implicated in the treatment of knee OA. Compression knee sleeves have been shown to provide mild stability and possibly improve proprioception [18-20]. Several studies have been conducted on the composition of knee sleeves. Germanium, a semiconductor metalloid, exhibits higher conductivity at certain temperatures, and it is hypothesized that its electrons would be released in a specific direction when faced with a certain temperature. A low-level observational study showed that fewer games were missed by players of an American major league soccer team who wore germanium-embedded knee sleeves [21]. Marino et al. demonstrated in a cohort study that germanium-embedded knee sleeves could improve clinical outcomes in patients with Kellgren and Lawrence grades one and two knee OA [22]. Elphingstone et al. carried out a small cohort study in which knee OA patients were given a knee sleeve that specifically uses synthetic fibers that incorporate finely processed, non-metallic, elemental semiconductor nanoparticles. The mechanism of action is thought to be the result of reflecting body heat as near-infrared light, which promotes vasodilation, improves nutrient delivery to the synovium, removes inflammatory mediators, and accelerates adenosine triphosphate production [23].
To date, however, no clinical studies have specifically examined knee sleeves containing far infrared ray-emitting materials. In sheets, graphene has semiconductor properties. When embedded with other semiconductor materials in a knee sleeve, a high emissivity is achieved, allowing for the reflection of far-infrared rays into the human body. This allows for dilation of blood vessels, thereby enhancing blood microcirculation and metabolism.
In this retrospective cohort study, we evaluated short-term patient-reported outcomes of conservatively managed OA patients after the use of a novel FIR-emitting compression knee sleeve. We hypothesize that this knee sleeve will provide clinically meaningful improvement in pain relief and function.
2. MATERIALS AND METHODS
This retrospective cohort study was conducted at a single urban institution from June 2024 to July 2024. A total of forty-eight patients undergoing nonoperative management for knee OA were fitted with 61 knee sleeves, 35 unilaterally and 26 bilaterally.
Due to loss to follow-up, 22 patients (30 total knees) completed both baseline and one-month follow-up assessments and were included in the final analysis. These patients were categorized into two groups based on chart review. Group 1 (HA-/Sleeve+) consisted of patients who received the FIR-emitting sleeve only and did not receive a Hyaluronic Acid (HA) injection at the time of fitting. Group 2 (HA+/Sleeve+) comprised patients who received both the FIR sleeve and an HA injection concurrently. Patients completed Patient-Reported Outcome Measures (PROMs) at baseline and four weeks post-treatment, including: the Knee Injury and Osteoarthritis Outcome Score, JR (KOOS, JR), Numeric Pain Rating Scale (NPRS), and a Five-Point Likert Satisfaction Scale.
Demographic data (age, sex, BMI) and clinical variables (unilateral vs. bilateral sleeve use, injection status) were extracted from the medical records. Data were analyzed at the sleeve level (n = 30), and each sleeve was considered an independent unit of analysis.
Descriptive statistics were used to summarize demographics and outcome measures. Continuous variables were reported as means with standard deviations. Due to the small sample size, no formal statistical comparisons were conducted between groups. Comparisons of baseline and follow-up PROMs within each group were demonstrated by calculating differences.
3. RESULTS
Patient demographics are summarized in Table 1. Of the 22 patients included in the final analysis, 67% were female with a mean age of 66 ± 11.942 years and a Body Mass Index (BMI) of 26.253 ± 5.412. Group one (HA-/Sleeve+) consisted of 12 knee sleeves fitted to ten patients (83% female), with a mean age of 59.583 ± 12.753 and BMI of 27.485 ± 6.675. Group two (HA+/Sleeve+) included 18 knee sleeves fitted to 12 patients (56% female) with a mean age of 70.278 ± 9.898 and BMI of 25.432 ± 4.398. Table 2 demonstrates that baseline patient-reported outcome measures between males and females were similar.
KOOS, JR evaluates knee health on a scale of 0 to 100. At baseline, the mean KOOS and JR scores for all patients were 57.603, Group one was 56.659, and Group two was 58.232. At one-month follow-up, the mean KOOS and JR scores were 68.929 compared to 68.041 in Group one and Group two, respectively Table 3. From baseline to one-month follow-up, there was a mean increase in KOOS and JR scores of 12.27 in Group one, and 9.809 in Group two (Fig. 1). While both groups show improvement, the HA-/Sleeve+ group demonstrated a numerically greater gain in knee health.
The Numerical Pain Rating Scale (NPRS) measures pain intensity on a scale from zero to ten, with zero indicating no pain and ten indicating the worst pain imaginable. At baseline, the mean pain score in Group one was six, compared to five in Group two. At one-month follow-up, the mean pain score for Group one and Group two was three Table 3. From baseline to one-month follow-up, patients in Group one had a three-point decrease in reported pain compared to a two-point decrease in Group two (Fig. 2). Overall, both groups experienced a decrease in pain, but there was a greater decrease in Group one.
|
Total N = 30 |
Group 1: HA-/Sleeve- N = 12 |
Group 2: HA+/Sleeve+ N = 18 |
|
|---|---|---|---|
| Age (mean years ± sd) | 66 ± 11.942 | 59.583 ± 12.753 | 70.278 ± 9.898 |
| BMI (mean ± sd) | 26.253 ± 5.412 | 27.485 ± 6.675 | 25.432 ± 4.398 |
|
Sex n (%) Female |
20 (67%) | 10 (83%) | 10 (56%) |
| Male | Female | Differences | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Total N = 10 |
Group 1: HA-/Sleeve+ N = 2 |
Group 2: HA+/Sleeve+ N = 8 |
Total N = 20 |
Group 1: HA-/Sleeve+ N = 10 |
Group 2: HA+/Sleeve+ N = 10 |
Total | Group 1: HA-/Sleeve+ | Group 2: HA+/Sleeve+ | |
| Knee Injury and Osteoarthritis Outcome Score, JR | 59.398± 15.565 | 51.701± 17.077 |
61.322± 15.769 |
56.705± 18.131 | 57.650± 13.178 |
55.761± 22.767 |
2.693 | 5.949 | 5.561 |
| Numeric Pain Rating Scale | 4.6± 2.41 |
6.5± 2.121 |
4.125± 2.357 |
5.25± 3.04 |
5.7± 3.129 |
4.8± 3.048 |
0.65 | 0.8 | 0.675 |
| 5-Point Likert Satisfaction Scale | 0.632± 0.342 | 0.5± 0.707 |
0.875± 0.641 |
0.7± 0.923 | 0.4± 0.516 |
1± 1.155 |
0.068 | 0.1 | 0.125 |
| Baseline | 1-month | Differences | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total |
Group 1: HA-/Sleeve+ |
Group 2: HA+/Sleeve+ | Total |
Group 1: HA-/Sleeve+ |
Group 2: HA+/Sleeve+ | Total | Group 1: HA-/Sleeve+ | Group 2: HA+/Sleeve+ | |
| Knee Injury and Osteoarthritis Outcome Score, JR | 57.603± 17.095 |
56.659± 13.189 |
58.232± 19.619 |
68.397± 13.75 |
68.929± 10.525 |
68.041± 15.828 |
10.794 | 12.27 | 9.809 |
| Numeric Pain Rating Scale | 5.033± 2.822 |
5.833± 2.918 |
4.5± 2.706 |
3± 2.477 |
3± 2.174 |
3± 2.722 |
2.033 | 2.833 | 1.5 |
| 5-Point Likert Satisfaction Scale | 0.733± 0.828 |
0.417± 0.515 |
0.944± 0.938 |
1.467± 1.106 |
1.833± 1.193 |
1.222± 1 |
0.734 | 1.416 | 0.278 |
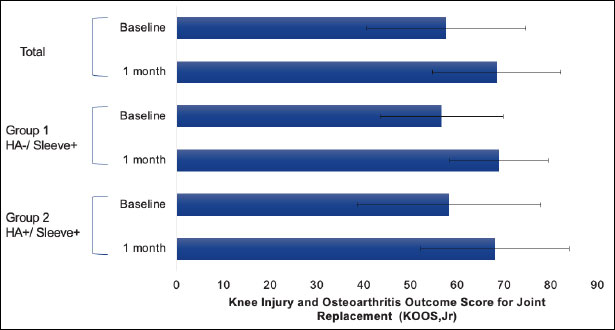
Knee health.
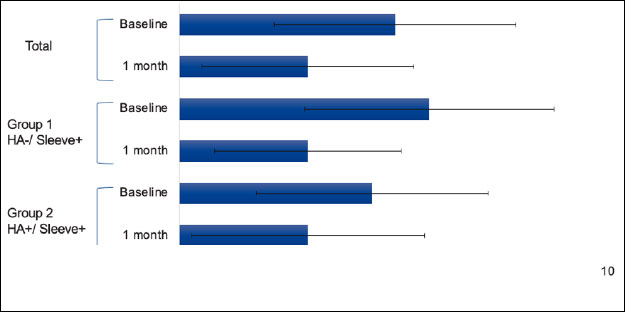
Knee pain.
The Five-Point Likert Satisfaction Scale assesses satisfaction and offers the following options: poor (zero), fair (one), good (two), very good (three), and excellent (four). At baseline, the mean reported satisfaction was 0.417 in Group one and 0.944 in Group two. At the one-month follow-up, the mean reported satisfaction was 1.833 in Group one and 1.222 in Group two Table 3. From baseline to one-month follow-up, there was a mean increase in reported satisfaction of 1.416 in Group one, and 0.278 in Group two (Fig. 3). In all groups, there was an improvement in satisfaction, with a greater improvement observed in Group one.
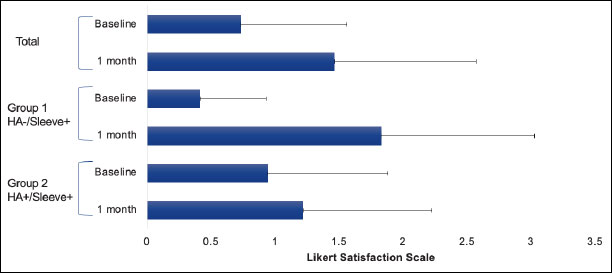
Knee satisfaction.
4. DISCUSSION
In this pilot study, we evaluated patient-reported outcomes following the use of a FIR-emitting compression knee sleeve in patients with knee OA, with or without concurrent HA injections. After one month, both groups demonstrated improvements in knee health, pain, and satisfaction, with greater numerical improvements observed in patients who received the sleeve alone (HA-/Sleeve+).
Prior studies have explored bioactive knee sleeves for OA management. Elphingstone et al. carried out a small cohort study in which knee OA patients wearing a bioactive knee sleeve showed improvement of at least 25 points in all KOOS scores (activities of daily living, pain, sports and recreation, symptoms, quality of life) from baseline to 1.5 months [23]. In contrast, our total patient population experienced a comparatively modest improvement of 10.794 points in the KOOS and JR scores from baseline to one-month follow-up. However, the average KOOS score at baseline for our total population was 57.603, whereas patients in the Elphingstone et al. study had a baseline of 41.04. Our patients had higher baseline KOOS and JR scores, indicating that they had better knee health and possibly less advanced knee osteoarthritis at the study’s onset. A change in scores of eight to ten points may represent the minimal perceptible clinical improvement of the KOOS [23, 24]. The KOOS JR values in all our groups showed improvements of over nine points, exceeding this threshold, suggesting meaningful clinical improvement.
Alternatively, it is possible that our timeline was too short to capture the longitudinal effects of the FIR sleeve and HA injections. However, Jivan et al. carried out a study measuring KOOS scores (activities of daily living, pain, sports and recreation, symptoms, quality of life) following HA injection. In their study, significant improvements in pain and sports and recreation metrics were observed at one month, suggesting that the effects of HA injection could be discerned within four weeks [25, 26]. Interestingly, in our study, patients who received HA injections did not show greater improvement than those who used the sleeve alone. Whether this reflects a lack of additive benefit, differences in disease severity, or variability in treatment response remains uncertain and warrants further investigation.
In the realm of managing knee osteoarthritis, the combined use of intra-articular injections and NSAIDs has been justified from a pharmacokinetic point of view to provide both rapid and long-term symptomatic benefit [25]. However, their undesired side-effect profiles are well-established. Additionally, in terms of efficacy, Soriano-Maldonado et al investigated the use of intra-articular cortisone injections administered two weeks prior to a 12-week PT program and found no difference in reducing pain sensitivity [27]. In the context of utilizing these modalities in tandem with knee compression, there is a lack of studies measuring synergistic combinations. It remains possible that a complementary effect between various treatments could reduce the overall pharmacological risk.
This pilot study has several limitations. First, the small sample size and retrospective design limit generalizability and preclude causal inference. The lack of a non-sleeve control group further limits the interpretation of the treatment effect. Second, adherence to sleeve use was not systematically monitored, which may have led to an underestimation of benefit. Third, the radiographic severity of OA was not recorded. The Kellgren-Lawrence grading system divides knee OA into grades based on its radiographic features that are associated with specific symptoms [28]. Future studies should incorporate Kellgren-Lawrence grading to allow for stratified analysis. Lastly, outcomes were assessed only at one month; longer follow-up is necessary to evaluate the durability of the effect.
CONCLUSION
Our data suggests that a FIR-emitting compression knee sleeve may be associated with improvements in knee health, reduced pain, and increased satisfaction among patients with knee OA. Although both groups demonstrated improvement, patients who received the knee sleeve without a concomitant HA injection appeared to experience greater gains across all outcome measures. This is the only clinical study specifically examining knee sleeves containing FIR-emitting materials. Future prospective studies with larger cohorts and controlled comparisons are needed to validate these preliminary results and determine the specific role of FIR knee sleeves in the nonoperative management of knee osteoarthritis.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: N.V.: Study conception and design; H.A., S.A., A.B.: Data collection; S.B., D.B., W.L., K.M.C., E.S.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| FIR | = Far Infrared Ray |
| OA | = Osteoarthritis |
| HA | = Hyaluronic Acid |
| NSAIDs | = Nonsteroidal Anti-Inflammatory Drugs |
| PROMs | = Patient-Reported Outcome Measures |
| KOOS, JR | = Knee Injury and Osteoarthritis Outcome Score, JR |
| NPRS | = Numeric Pain Rating Scale |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethical committee of the Hospital for Special Surgery (2024-1531).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all individual participants included in the study.
AVAILABILITY OF DATA AND MATERIAL
All data generated or analyzed during this study are included in this published article.
ACKNOWLEDGEMENTS
Declared none.


