RESEARCH ARTICLE
Optimal Treatment Interval of Viscosupplementation for Osteoarthritic Knee Pain: Real-world Evidence from a Retrospective Study
Janice Johnston1, Jeffrey Muir2, *, Michael J. Sloniewsky3
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
E-location ID: e187432502212020
Publisher ID: e187432502212020
DOI: 10.2174/18743250-v16-e221202-2022-6
Article History:
Received Date: 28/12/2022Revision Received Date: 25/10/2022
Acceptance Date: 2/11/2022
Electronic publication date: 29/12/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
The evidence supporting multiple courses of viscosupplementation for knee osteoarthritis continues to grow; however, the optimal treatment interval for repeat courses is not well understood. To address this, we compared baseline pain and disability scores in patients returning for subsequent treatment with their prior discharge scores.
Methods:
We retrospectively collected data from patients at 16 rehabilitation clinics who presented for repeated courses of viscosupplementation treatment for knee OA. Primary outcomes were pain (visual analog scale, VAS) and Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores, which were collected following the initial treatment course and compared with scores upon return for treatment. The proportion of patients who fulfilled a minimal clinically important difference in each outcome was calculated.
Results:
61 patients (81 knees) were included in our analysis. After a 6-month treatment interval, no significant differences were noted between post-discharge and returning scores for either VAS (p=0.73) or WOMAC (Pain: p=0.42; Function: p=0.54; Stiffness: p=0.29). Patients waiting 9 months to return for treatment saw a 45% increase in their pain scores (p=0.10) and significant worsening in WOMAC scores (Pain: p=0.007; Function: p=0.03; Stiffness: p=0.04). At 12 months, pain (p=0.01), WOMAC Pain (p=0.05), and WOMAC Stiffness (p=0.02) had all worsened significantly compared to discharge following the initial course.
Conclusion:
Our data indicate that patients who return for treatment within a 6-month treatment interval maintain their improvements, but that when the interval increases to 9 months or more, patients present as significantly worsened, having lost the benefit of their initial course of treatment.
1. INTRODUCTION
Knee osteoarthritis (OA) remains a prevalent and potentially debilitating condition, affecting millions worldwide each year [1] and resulting in the ongoing development of treatment modalities to address the accompanying pain and disability. For patients with early- to mid-stage knee OA, viscosupplementation (VS) offers a safe and effective treatment option that can significantly decrease pain and improve function and quality of life [2-4].
The efficacy of VS for OA is now supported by decades of clinical studies that have consistently demonstrated a significant treatment effect. Beneficial results from VS are supported by systematic reviews and meta-analyses, with single courses of treatment associated with significant and clinically important improvements in pain and function [5-8]. As its use has continued, additional evidence has shed important light on the benefit of multiple courses of VS, with several studies demonstrating either maintenance of improvements realized after an initial course [9, 10] or continued improvement with repeated courses [4, 11-13]. Current treatment guideline recommendations support the administration of both single and repeat courses of treatment for symptomatic patients [3, 14, 15]. The growing evidence of the value of VS for knee OA has also shaped the willingness of payers to reimburse patients for VS treatment, with the current evidence informing not only the number and timing of injections for each single course of treatment, but also the interval between courses of treatment for patients who receive repeated courses. Currently, reimbursement for repeated courses of treatment is based on a 6-month treatment interval [16-18]. The data supporting the current approach to multiple courses of treatment, however, are based on establishing a minimum time between courses of treatment. A question of equal importance is that of the maximum time between courses, and how prolonging the treatment interval affects the treatment effect. The question relevant to patients, practitioners, and policymakers is: how long is too long to wait to return for treatment?
The evidence supporting multiple courses of treatment generally involves studies where a fixed treatment interval was used. There is less evidence evaluating the relative effectiveness of repeated courses of treatment based on variable treatment intervals. The question of the optimal time between treatment courses is crucial, as it informs how multiple courses should be designed to minimize any loss of treatment effect without over-treating the patient. To investigate this, we analyzed real-world data from patients who underwent repeat courses of VS treatment for knee OA, without a regimented treatment interval.
2. MATERIALS AND METHODS
2.1. Study Design
This was a retrospective, observational, longitudinal cohort study of data collected from patients undergoing VS treatment for knee OA, conducted at 16 rehabilitation clinics across the United States. The study conformed to the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) guidelines for observational studies [19]. Data collection complied with the Declaration of Helsinki [20] and informed consent from patients and ethics approval (Advarra IRB) were obtained prior to data collection. This analysis was performed as part of a larger, longitudinal study examining the effect of repeat courses of VS on patient outcomes in knee OA [21]. Patient eligibility and data collection in this study are based on the initial study, with a convenience sample of eligible patients used in this analysis. The current analysis examined the impact of treatment interval on patient outcomes (pain and functional abilities).
2.2. Study Population
Patient eligibility for this analysis was based on the criteria set out in the initial study, which included:
(1) Treated with VS for primary knee OA between January 2014 and June 2020,
(2) A confirmed diagnosis of OA, based on the Medicare Local Coverage Determination rules [22]: physical examination, standing radiographs, a detailed medical history, and patient-reported pain in the affected knee that interferes with basic activities of daily living (ADLs), and
(3) Clinical outcomes recorded at baseline (i.e., prior to the first injection) and one week following the final injection for each course of treatment.
Additionally, patients were eligible to be included in the current analysis if they completed one full course of VS, administered over a standard period of 6-8 weeks, and returned for a subsequent treatment course of treatment. No specific criteria were applied regarding patients’ return for treatment. Patients returned for the subsequent course of their own volition, based on their symptom status, i.e., the perceived increase in pain or decrease in functional abilities. Visual analog scale (VAS) and Western Ontario and McMaster Arthritis Index (WOMAC) scores were recorded on each patient’s return to provide baseline scores for the second course of treatment. The treatment interval was defined as the time between the post-treatment assessment that followed the initial course of treatment and the date that baseline scores were recorded for the subsequent course of treatment.
2.3. Outcomes
The primary outcomes for this study included: pain, assessed via VAS (0-10) and WOMAC scores for all domains (pain, physical function, and stiffness). Demographic data was recorded for all patients including patient age, sex, Kellgren-Lawrence (KL) score, treatment date, body mass index (BMI), and treated knee (i.e., right, left, or bilateral).
Data for each knee treated was recorded at baseline (prior to the course of treatment initiation) and one week following the completion of each course of treatment. Patients categorized as “bilateral” received treatment for both knees at different time points during the study period. In these cases, data was collected for each knee separately and patients were categorized as “bilateral” for demographic purposes only.
2.4. Treatment Regimen
Each VS course was administered as a series of intra-articular injections at weekly intervals, totaling 5 injections, over a period of 6-8 weeks. In this real-world study, no restrictions were placed on the time between courses, or the number of courses received. Further, no restrictions or limitations were placed on the patients regarding physiotherapy, rehabilitation, or medication (analgesic or otherwise). Each patient’s treatment regimen – beyond the number of injections and timeframe for each course of treatment – was at the discretion of the treating physician. The VS formulations administered were: Genvisc850 (25 mg/2.5 mL, OrthogenRx, Inc., Doylestown, PA, USA), Supartz (25 mg/2.5 mL, Bioventus LLC, Durham, NC, USA), and Orthovisc (30 mg/2 mL, Pendopharm, Montreal, PQ, Canada).
2.5. Data Analysis and Statistics
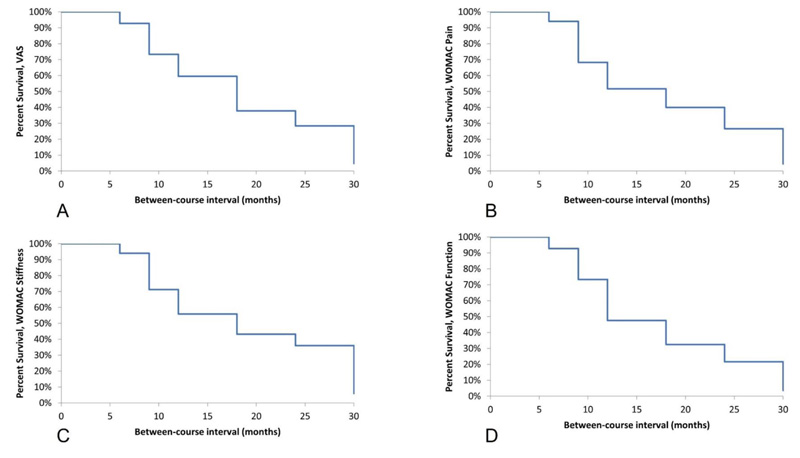
Patients were stratified based on the time between courses of treatment. Pain and function scores following the completion of each course of treatment were then compared to baseline scores upon each patient’s return for subsequent treatment to determine the change in pain or function status, if any, that occurred during the treatment interval. Outcomes scores are presented as mean (standard deviation) or mean ± standard deviation and mean scores were compared based on treatment interval using student’s t-test or single-factor analysis of variance (ANOVA), as appropriate. Categorical variables were compared using the chi-squared test or Fisher’s exact test as appropriate. Responder rates were calculated based on whether a minimal clinically important difference (MCID) was reported for each outcome measure when comparing post-treatment scores with baseline scores. MCID was also used to determine the degree of worsening in patients upon their return for treatment [23-25]. For VAS scores, a change of 30% was considered clinically important [26], while for WOMAC scores, a change of 20% was considered clinically important [27]. Kaplan-Meier survival analysis was used to examine the effect of treatment interval on the proportion of patients who maintained their treatment effect following their initial course of treatment.
3. RESULTS
3.1. Descriptive Statistics
A total of 61 patients (81 knees) were included in this analysis. The mean age of participants was 70.9±9.5 years, with a mean BMI of 32.2±7.6. Females comprised 61% (37/61) of the study population. GenVisc850 was the most commonly prescribed formulation (47/61, 77%), followed by Supartz (11/61, 18%) and Orthovisc (3/61, 5%). GenVisc850 and Supartz are administered as once-weekly treatments for 5 consecutive weeks while Orthovisc is administered once weekly for 3 weeks. Our study included too few patients receiving Orthovisc to perform any meaningful comparisons based on the formulation administered. No significant differences were noted in the demographic statistics when participants were stratified based on the treatment interval (Table 1).
3.2. Effect of Treatment Interval on Pain Scores
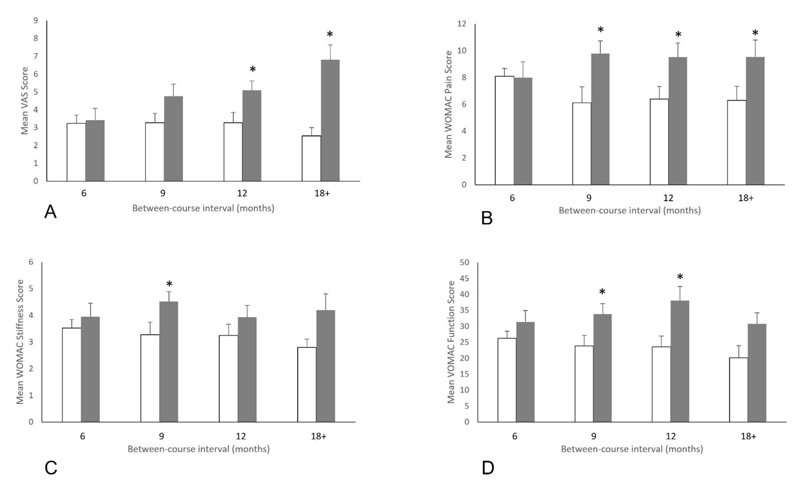
Upon return following an initial course of treatment, all patients reported higher baseline VAS scores when compared with scores following their initial course of treatment. The pattern of this change indicated that as the treatment interval increased, so did the patient’s baseline score upon return, with a survival analysis indicating that treatment effects had decreased mildly at 6 months but began to decrease significantly at 9 months and beyond (Fig. 1A). Patients returning after 6 months reported a 6% increase in VAS scores (3.4±3.0 vs. 3.2±2.4, p=0.73), which increased to 45% (4.8±3.4 vs. 3.3±2.9, p=0.10) when the treatment interval was 9 months, to 56% (5.1±3.3 vs. 3.3±2.9, p=0.01) when returning after 12 months and to 167% (6.8±2.7 vs. 2.5±3.0, p=0.004) when patients returned after 18 months or longer (Fig. 2A).
In patients who returned after 6 months, the mean increase in VAS scores represented a clinically important change in 29% of cases (6/21). This proportion increased as the time between courses increased (Fig. 2A). In patients who returned after 9 months (52%, 13/25, p=0.11 vs. 6-month interval) and 12 months (47%, 7/15, p=0.27 vs. 6-month interval), more patients saw a clinically important increase in their pain scores, although these differences were not significant. At ≥18 months, significantly more patients had reported a clinically important increase in pain scores (76%, 16/21, p=0.02 vs. 6 months) (Table 2).
| Treatment interval | ||||||
|---|---|---|---|---|---|---|
| Full cohort | 6 months | 9 months | 12 months | ≥18 months | p-value | |
| Age, mean (SD) | 67.2 (13.1) | 67.0 (9.7) | 71.1 (8.3) | 71.3 (10.9) | 70.2 (10.5) | 0.41a |
| BMI, mean (SD) | 32.2 (7.6) | 31.5 (5.1) | 31.4 (7.6) | 32.3 (8.1) | 33.1 (7.7) | 0.21a |
| Kellgren-Lawrence score, n (%)0 1 2 3 4 |
18 (38) 1 (2) 12 (26) 24 (51) 6 (13) |
6 (43) 0 (0) 5 (36) 3 (21) 0 (0) |
5 (25) 0 (0) 3 (15) 9 (45) 3 (15) |
3 (25) 1 (8) 2 (17) 6 (50) 0 (0) |
4 (27) 0 (0) 2 (13) 6 (40) 3 (20) |
0.31b |
| Sex, n (%) M F |
24 (39) 37 (61) |
7 (50) 7 (50) |
6 (30) 14 (70) |
5 (42) 7 (58) |
6 (40) 9 (60) |
0.70b |
| Side, n (%) R L Bi |
21 (34) 20 (33) 20 (33) |
5 (36) 3 (21) 6 (43) |
6 (30) 9 (45) 5 (25) |
5 (42) 4 (33) 3 (25) |
5 (33) 4 (27) 6 (40) |
0.78b |
| Supplement, n (%) GenVisc850 Supartz Orthovisc |
47 (77) 11 (18) 3 (5) |
12 (86) 2 (14) 0 (0) |
17 (85) 2 (10) 1 (5) |
7 (58) 4 (33) 1 (8) |
11 (73) 3 (20) 1 (7) |
0.63b |
b. Chi-square or Fisher-Freeman-Halton test (based on statistical appropriateness) based on treatment interval
| - | Treatment Interval | |||
|---|---|---|---|---|
| 6 months | 9 months | 12 months | ≥18 months | |
| VAS, n (%) | 6 (29) | 13 (52) | 7 (47) | 16 (76)* |
| WOMAC Pain, n (%) | 5 (24) | 17 (68)* | 9 (60)* | 14 (64)* |
| WOMAC Function, n (%) | 5 (24) | 15 (60)* | 8 (53) | 12 (55)* |
| WOMAC Stiffness, n (%) | 6 (29) | 13 (52) | 13 (87)* | 16 (73)* |
3.3. Effect of Treatment Interval on WOMAC Scores
WOMAC scores followed a similar pattern to pain scores, with scores generally worsening during the treatment interval, and with the degree of worsening being greater as the treatment interval increased. For WOMAC Pain, survival analysis indicated a mild worsening of scores at 6 months, but significant worsening beginning at 9 months (Fig. 1B). Scores for patients returning after 6 months were essentially unchanged compared to their post-treatment scores (8.0±5.1 on return vs. 8.1±5.5 after the initial course, p=0.42). For patients returning after 9, 12, and ≥18 months, the score on return was significantly higher than following their initial course of treatment. Returning scores were 60% higher (9.8±4.6 vs. 6.1±4.6, p=0.007) at 9 months, 49% higher (9.5±4.5 vs. 6.4±4.0, p=0.05) at 12 months and 52% higher (9.5±4.6 vs. 6.3±4.0, p=0.02) at 18 months (Fig. 2B).
Amongst patients who returned for treatment after 6 months, 24% reported a clinically important increase in their WOMAC Pain scores (5/21). This proportion was significantly higher at 9 months (68%, 17/25, p=0.003 vs. 6 months) and 12 months (60%, 9/15, p=0.03 vs. 6 months), and remained significant at ≥18 months (64%, 14/22, p=0.008) (Table 2 and Fig. 2B).
Scores for WOMAC Function and Stiffness followed a similar trend to pain scores, with the survival analysis for both domains indicating a small worsening at 6 months but a significant worsening when the treatment interval was 9 months or greater (Fig. 1C and 1D). Patients who returned after 6 months reported increased scores, although these differences were not statistically significant compared with their initial discharge scores (Function: 4.0±2.3 vs. 3.5±2.1, p=0.54; Stiffness: 31.4±16.1 vs. 26.3±15.0, p=0.29). At 9 months, the increase in both scores were statistically significant compared to scores following the initial course of treatment (Function: 38% increase, 4.5±1.9 vs. 3.3±2.1, p=0.03; Stiffness: 42% increase, 33.8±16.5 vs. 23.9±16.7, p=0.04). At 12 months, WOMAC Function scores were significantly higher upon return (38.1±17.3 vs. 23.6±14.5, p=0.02), but at 18 months and beyond, returning scores, while higher, did not reach statistical significance. For WOMAC Stiffness, scores for patients returning at 12 months (61% increase, 38.1±17.3 vs. 23.6±14.5, p=0.02) were significantly higher than following the initial course of treatment while at ≥18 months, returning scores trended towards higher but did not reach statistical significance (53% increase, 30.8±16.1 vs. 20.2±10.3, p=0.07) (Fig. 1C and 1D).
Patients returning after 6 months, 24% and 29%, respectively, reported an increase in Function and Stiffness scores that was clinically important (5/21, 6/21). This proportion increased to 60% for Function at 9 months (15/25, p=0.01 vs. 6 months) and remained higher for those returning after 12 months (53%, 8/15, p=0.07 vs. 6 months) and ≥18 months (73%, 16/22, p=0.02). WOMAC Stiffness scores for patients returning at 9 months saw a clinically important increase in 52% (13/25, p=0.11) of patients, while among those returning at 12 months (87%, 13/15, p=0.0006) and ≥18 months (64%, 12/22, p=0.008), significantly greater proportions reported a clinically important change when compared to those returning at 6 months (Table 2, Fig. 2C and 2D).
4. DISCUSSION
Viscosupplementation remains a safe and effective treatment for early- to mid-stage osteoarthritis of the knee. In general, a 6-month window between courses is viewed as the most appropriate timeframe for patients receiving multiple courses of treatment. While this treatment interval is widely supported in the literature, what is less well understood is how extending this timeframe beyond 6 months impacts treatment effects. We examined real-world data from a long-term study to determine the impact of varying treatment intervals on pain and function scores. Our results concur with the literature and indicate that with an interval of 6 months, treatment effects are well maintained, but found that in patients who waited 9 months or more to return for treatment, VAS and all domains of WOMAC scores worsened significantly and the benefits of the initial course of treatment were partially lost.
Our study noted no significant worsening of pain or function scores when patients returned after 6 months, observing that both VAS and all domains of WOMAC scores were maintained to the point of being essentially unchanged between discharge from the initial course of treatment and their return for retreatment. These observations align with the consensus within the field that short-term improvement (≤6 months) can be expected following an initial course of viscosupplementation, but that this improvement wanes after this initial period. Several systematic reviews [5, 8, 28, 29] and large-scale studies [30] have and have all observed a 6-month post-treatment window where symptoms are abated. Indeed, this expectation is reflected in current reimbursement guidelines, which do not support coverage of treatment in intervals shorter than 6 months [17]. Our study supports these conclusions but also provides new evidence regarding the relative impact of prolonging the treatment interval greater than these recommendations, as we noted substantial increases in both pain and WOMAC scores in patients who extended their treatment interval beyond 6 months. We were unable to identify similar studies examining variations in treatment interval. Our study thus provides initial evidence that prolonging the treatment interval may result in loss of treatment effect and may be beneficial in setting expectations of patients regarding expected improvement duration following an initial course of treatment. Further research on the implications of prolonging the treatment interval is warranted.
Interestingly, there were demographic differences within our cohort that may explain this observation. Patients returning after 6 months were younger than those returning after either 9, 12, or 18 months, suggesting that age may be a factor. When patients returning after 6 months were age-matched with those returning after 9 months and VAS scores compared, scores at 9 months remained higher, although not significantly (3.3±3.7 vs. 4.8±3.4, p=0.22). Similarly, patients returning after 6 months had an average Kellgren-Lawrence score of 1.4±1.3, which was lower than those returning after 9 (2.3±1.4, p=0.07 vs. 6 months), 12 (1.9±1.3, p=0.28) or 18 (2.7±1.5, p=0.06) months. BMI, however, did not appear to play a role, as there were no differences between mean BMI in patients stratified based on their treatment interval (Table 2). The results of our multivariate analysis, however, do not completely support these observations, as only the Kellgren-Lawrence score was noted to be a significant predictor of returning VAS or WOMAC scores, suggesting that these variables independently are not indicative of returning scores and that multiple factors combine to determine when patients return for subsequent treatment. This is an interesting finding, as current guidelines consider disease severity, age, and BMI as part of their recommendations [3, 14, 15], with an expectation that younger age and lower severity of disease are indicators of treatment success [31]. However, there is also evidence indicating that higher BMI is also associated with poorer treatment response, including one recent study that examined the independent and combined effects of disease severity and BMI on treatment response, noting that both were significant contributors [32]. Collectively, the data suggest a role for age and OA severity as factors when considering the optimal treatment interval. This observation warrants further investigation.
Our study has limitations, primarily the small sample size of patients who returned 18 months or more following their initial course of treatment. As patients returned of their own volition, there is the possibility that variables other than pain or dysfunction (i.e., availability, scheduling, co-morbidities, illness) could have extended their treatment interval. Further, patients who had a less favourable response to treatment after their initial course may not have been as likely to return promptly for a second course. As such, there may be an element of selection bias at play in our study. Combined with the nature of the study (retrospective design, convenience sample), these factors may be considered limitations that limit the generalizability of the results. Further, the design of our analysis, i.e., a sub-group analysis performed as part of a larger study that established the study parameters may be somewhat limiting. However, the real-world setting of our study and its multicenter design provide valuable data regarding the impact of varying treatment intervals, data that may not be available if it were collected prospectively due to more stringent study and treatment protocols. Future studies with larger sample sizes should help to augment our results and add to the applicability of the findings. Finally, our study focused on patient-reported outcomes which, while increasingly important as part of healthcare decision-making processes, are nonetheless subjective in nature. Future studies will include objective data collected at the treatment interval to provide additional data.
CONCLUSION
Our review of real-world data indicates that, for patients with early- to mid-stage knee OA, improvements in pain and function following an initial course of viscosupplementation are maintained in the 6 months following treatment, but that improvements in pain levels begin to deteriorate at 9 months and are significantly worsened at 12 months. Functional abilities scores are similarly maintained at 6 months but are significantly worsened at 9 months, an observation that continues at 12 months and beyond.
LIST OF ABBREVIATIONS
| VS | = Viscosupplementation |
| STROBE | = Strengthening the Reporting of Observational Studies in Epidemiology |
| ADLs | = Activities of Daily Living |
| WOMAC | = Western Ontario and McMaster Arthritis Index |
| VAS | = Visual Analog Scale |
| KL | = Kellgren-Lawrence |
| BMI | = Body Mass Index |
| MCID | = Minimal Clinically Important Difference |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study is ethically approved by Advarra IRB and informed consent was obtained prior to data collection.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
Dr. Muir received consultancy fees from RMG Holdings for the completion of this work. No other funding was associated with this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020; 29-30: 100587. |
| [2] | Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review. Semin Arthritis Rheum 2019; 48(4): 563-72. |
| [3] | Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016; 45(4)(Suppl.): S28-33. |
| [4] | Strand V, Lim S, Takamura J. Evidence for safety of retreatment with a single intra-articular injection of Gel-200 for treatment of osteoarthritis of the knee from the double-blind pivotal and open-label retreatment clinical trials. BMC Musculoskelet Disord 2016; 17(1): 240. |
| [5] | Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: A systematic review. Semin Arthritis Rheum 2018; 48(2): 168-75. |
| [6] | Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Libr 2006; 2014(11): CD005321. |
| [7] | Concoff A, Sancheti P, Niazi F, Shaw P, Rosen J. The efficacy of multiple versus single hyaluronic acid injections: a systematic review and meta-analysis. BMC Musculoskelet Disord 2017; 18(1): 542. |
| [8] | Tapasvi S, Mohanty SS, Vedavyasa Acharya KK, Bhattacharya K, Easwaran R, Charugulla SN. Viscosupplementation for management of knee osteoarthritis from an indian perspective: An expert consensus report. Pain Ther 2019; 8(2): 217-31. |
| [9] | Abate M, Vanni D, Pantalone A, Salini V. Hyaluronic acid in knee osteoarthritis: Preliminary results using a four months administration schedule. Int J Rheum Dis 2017; 20(2): 199-202. |
| [10] | Altman RD, Rosen JE, Bloch DA, Hatoum HT. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: Results of the open-label extension study of the flexx trial. Osteoarthritis Cartilage 2011; 19(10): 1169-75. |
| [11] | Heger R, Paulsen G, Fickert U, Kresmann M. Open-label study of initial and repeat treatment cycles of hylan G-F 20 in patients with symptomatic knee Osteoarthritis. Open Rheumatol J 2016; 10(1): 88-100. |
| [12] | Benazzo F, Perticarini L, Padolino A, et al. A multi-centre, open label, long-term follow-up study to evaluate the benefits of a new viscoelastic hydrogel (Hymovis®) in the treatment of knee osteoarthritis. Eur Rev Med Pharmacol Sci 2016; 20(5): 959-68. |
| [13] | Kolarz G, Kotz R, Hochmayer I. Long-term benefits and repeated treatment cycles of intra-articular sodium hyaluronate (Hyalgan) in patients with osteoarthritis of the knee. Semin Arthritis Rheum 2003; 32(5): 310-9. |
| [14] | Raman R, Henrotin Y, Chevalier X, et al. Decision algorithms for the retreatment with viscosupplementation in patients suffering from knee Osteoarthritis: Recommendations from the European viscosupplementation consensus group (EUROVISCO). Cartilage 2018; 9(3): 263-75. |
| [15] | Viscosupplementation for Knee Osteoarthritis. A Review of Clinical and Cost-Effectiveness and Guidelines. Ottawa: CADTH 2017. |
| [16] | Bedenbaugh AV, Bonafede M, Marchlewicz EH, Lee V, Tambiah J. Real-world health care resource utilization and costs among US patients with knee osteoarthritis compared with controls. Clinicoecon Outcomes Res 2021; 13: 421-35. |
| [17] | Bhadra AK, Altman R, Dasa V, et al. Appropriate use criteria for hyaluronic acid in the treatment of knee Osteoarthritis in the United States. Cartilage 2017; 8(3): 234-54. |
| [18] | McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22(3): 363-88. |
| [19] | Strengthening the reporting of observational studies in epidemiology 2022. Available from: https://www.strobe-statement.org/ |
| [20] | World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310(20): 2191-4. |
| [21] | Johnston J, Brown K, Muir J, Sloniewsky MJ. Long-term outcomes of single versus multiple courses of viscosupplementation for Osteoarthritic knee pain: Real-world, multi-practice experience over a six-year period. J Pain Res 2021; 14: 2413-21. |
| [22] | CMS.gov. Local Coverage Determinations 2021. Available from: https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs |
| [23] | Curtis JR, Yang S, Chen L, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid Arthritis patients. Arthritis Care Res (Hoboken) 2015; 67(10): 1345-53. |
| [24] | Yeo F, Ng CC, Loh KWJ, et al. Minimal clinically important difference of the EORTC QLQ-CIPN20 for worsening peripheral neuropathy in patients receiving neurotoxic chemotherapy. Support Care Cancer 2019; 27(12): 4753-62. |
| [25] | Revicki DA, Cella D, Hays RD, Sloan JA, Lenderking WR, Aaronson NK. Responsiveness and minimal important differences for patient reported outcomes. Health Qual Life Outcomes 2006; 4(1): 70. |
| [26] | Lee JS, Hobden E, Stiell IG, Wells GA. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med 2003; 10(10): 1128-30. |
| [27] | Maredupaka S, Meshram P, Chatte M, Kim WH, Kim TK. Minimal clinically important difference of commonly used patient-reported outcome measures in total knee arthroplasty: review of terminologies, methods and proposed values. Knee Surg Relat Res 2020; 32(1): 19. |
| [28] | Trigkilidas D, Anand A. The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. Ann R Coll Surg Engl 2013; 95(8): 545-51. |
| [29] | Bannuru RR, Brodie CR, Sullivan MC, McAlindon TE. Safety of repeated injections of sodium hyaluronate (SUPARTZ) for knee Osteoarthritis. Cartilage 2016; 7(4): 322-32. |
| [30] | Navarro-Sarabia F, Coronel P, Collantes E, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee Osteoarthritis: The AMELIA project. Ann Rheum Dis 2011; 70(11): 1957-62. |
| [31] | Uçar D, Dıraçoğlu D, Süleyman T, Çapan N. Intra-articular hyaluronic acid as treatment in elderly and middle-aged patients with knee osteoarthritis. Open Rheumatol J 2013; 7(1): 38-41. |
| [32] | Conrozier T, Eymard F, Chouk M, Chevalier X. Impact of obesity, structural severity and their combination on the efficacy of viscosupplementation in patients with knee osteoarthritis. BMC Musculoskelet Disord 2019; 20(1): 376. |