All published articles of this journal are available on ScienceDirect.
Mortality Risk Assessment of Total Knee Arthroplasty and Related Surgery After Percutaneous Coronary Intervention
Abstract
Background:
The optimal antiplatelet therapy (APT) treatment strategy after Coronary Artery Stenting (CAS) in non-cardiac surgery, such as total knee arthroplasty (TKA) or urgent TKA-related surgery remains unknown.
Methods:
We built a decision tree model to examine the mortality outcomes of two alternative strategies for APT after CAS use in the perioperative period namely, continuous use and discontinuation.
Results:
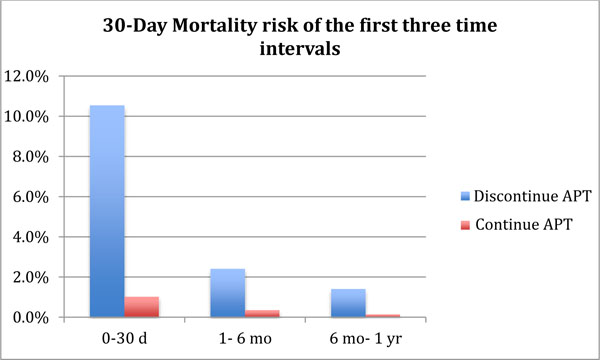
If surgery was performed in the first month after CAS placement, discontinuing APT led to an estimated 30-day post TKA mortality of 10.5%, compared to 1.0% in a strategy with continuous APT use. Mortality with both strategies decreased with longer intervals.
Conclusion:
Our model demonstrated that APT discontinuation in patients undergoing TKA or urgent TKA related surgery after CAS placement might lead to greater 30-day mortality up to one year.
INTRODUCTION
In 1990, bare-metal coronary artery stent (CAS) implantation was introduced and revolutionized percutaneous coronary intervention. It significantly reduced abrupt vessel closure and restenosis after balloon angioplasty [1]. Drug-eluting stents were developed to address the neointimal hyperplasia at the site of bare-metal stent implantation that often caused restenosis [2]. The endothelial trauma caused by the implantation of a stent makes anticoagulation therapy with antiplatelet medication necessary to prevent intraluminal thrombosis. Over 1,000,000 coronary interventions are performed annually in the United States [3]. Non-cardiac surgery has been documented to occur in at least 5% of stent recipients within the first year after CAS placement [4, 5]. It has been reported that of the patients undergoing CAS placement, orthopaedic procedures account for approximately 20-33% of all subsequent non-cardiac surgeries [5, 6]. Among all orthopaedic procedures, total knee arthroplasty (TKA) remains one of the most commonly performed surgeries and its utilization is increasing [7].
Dual antiplatelet therapy, typically consisting of either thienopyridines (clopidogrel [Plavix, Sanofi-Aventis, Bridgewater, NJ] or ticlopidine [Ticlid, Hoffmann-LA Roche Inc, Nutley, NJ]) in combination with aspirin therapy, has been recommended in the prevention of early artery stent thrombosis. It is, however, often a concern to the orthopaedic surgeon performing a procedure due to the increased bleeding risk [8].
The management of anticoagulation among recipients of CAS who undergo non-cardiac procedures, such as TKA, has not been standardized and remains controversial. The American College of Cardiology/American Heart Association practice guidelines recommend continuing thienopyridine therapy plus aspirin for up to a year, depending on the type of the stent, to avoid stent thrombosis and restenosis [9, 10]. However, patients who experience significant quality of life reduction due to symptomatic knee osteoarthritis or in the setting of an urgent TKA related complication such as periprosthetic fracture may not able to wait until the one-year time interval has elapsed. Due to the known high morbidity of hematoma formation and the possibility of infection after TKA or TKA related surgery, orthopaedic surgeons may opt for cessation of oral antiplatelet therapy (APT) therapy. To our knowledge, there are no treatment guidelines that aid in the decision making of perioperative APT management in the setting of TKA or TKA related procedures.
We therefore undertook a model-based evaluation of 30-day mortality post TKA or urgent TKA related surgery using the available published literature parameters to quantify the trade-offs between a potential postoperative hematoma and subsequent infection and mortality risk due to stent thrombosis that is closely associated with premature cessation of antiplatelet agents.
We used a decision analysis model that is a useful tool in assisting physicians to determine the best clinical management option in uncertain areas. It is useful since it provides a more structured approach to evidence- based decisions [11].
METHODS
Analytic Overview
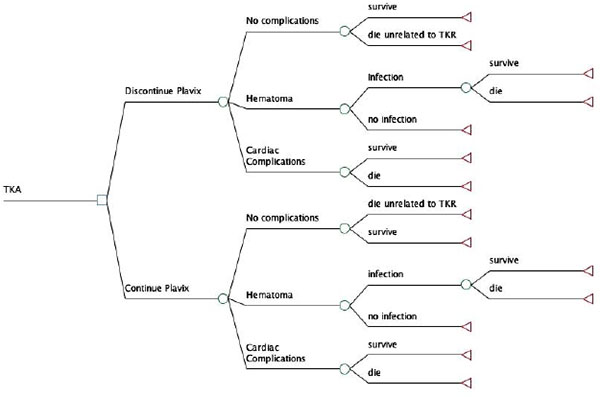
We constructed a decision tree to discern the 30- day mortality in patients undergoing either primary TKA surgery or urgent TKA related surgery for complications such as periprosthetic infection or periprosthetic fracture. Since mortality decreases drastically with a longer duration between CAS placement and TKA, we conducted three stratified analyses according to the time interval between CAS placement and TKA or TKA related surgery: 0-30 days, 1 month- 6 months and 6 months- 1 year (Fig. 1). We considered two alternative treatment strategies for APT: 1) leaving patients on APT (continuous APT), or 2) taking patients off APT in the perioperative period of TKA or TKA-related surgery (discontinued APT). The APT regimen consisted of aspirin in combination with either thienopyridine (Clopidogrel [Plavix, sanofi-aventis, Bridgewater, NJ] or ticlopidine [Ticlid, Hoffmann-LA Roche Inc, Nutley, NJ]). Patients in the first treatment group received both Plavix and Aspirin perioperatively without discontinuation. The second treatment group discontinued aspirin and Plavix pre-operatively, at least three days prior to the operative intervention and was restarted most commonly on the first postoperative day. All transition probabilities were derived from the published literature or estimated using expert opinion when data were not available. The analysis was conducted using a commercially available decision analysis software package (TreeAge Pro 11, TreeAge software, Williamstown, MA).
Model Structure
Our decision tree is depicted in Fig. (2). The principal decision consisted of either discontinuation or continuation of the APT prior to TKA or TKA-related surgery in a patient who underwent prior CAS placement. The analysis was conducted separately for each of the three different time intervals between CAS placement and TKA. In the model, after the prosthesis implantation, patients either had a successful postoperative course without any complications or suffered from hematoma formation or cardiac complications. Hematoma formation after a TKA does not only cause pain and possible stiffness but is a major source for infection [12, 13]. Cardiac complications, such as stent thrombosis that lead to myocardial infarction, carry a well-recognized high mortality rate. It was assumed that the chance of infection after hematoma formation in the knee would be the same for both treatment strategies.
Decision analysis is an analytic approach that quantifies the benefits and adverse events of alternative treatment strategies in a probabilistic manner using published data as input parameters. Impact of uncertainty in input parameters on outcomes is evaluated in sensitivity analysis [14].

Model Parameters
Population
We considered a hypothetical cohort of individuals with advanced osteoarthritis requiring a primary TKA or surgery to address complication of TKA such as periprosthetic infection or periprosthetic fracture.
Base Case
Transition probabilities used in the model are shown in Table 1. Bleeding after TKA was defined as hematoma that required surgical evacuation or as moderate postoperative bleeding using the American College of Cardiology/ American Heart Association guidelines [15]. Rates of deep infection after hematoma (11.9%) were derived from Galat et al. [12] The mortality associated with periprosthetic infection was derived from Cierny et al. [16]
| Strategy | Time interval between CAS and TKA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0-30 d | 1- 6 mo | 6 mo- 1 yr | Range (%) | Reference: | |||||||
| Discontinue APT | Hematoma/ bleeding |
31.0 | 9.3 | 2.4 | 0.2- 31.0 | [17-19, 41] | |||||
| Cardiac Event | 23.2 | 9.5 | 7.0 | 6.0-37.5 | [17, 20, 27-29, 42] | ||||||
| Death related to Cardiac Event | 44.9 | 24.9 | 19.8 | 0.2-85.7 | [6, 17, 22, 28, 34, 35, 42-44] | ||||||
| Continue of APT | Hematoma/ bleeding | 26.1 | 21.0 | 9.5 | 9.5- 43.8 | [20-25] | |||||
| Cardiac Event | 7.4 | 3.2 | 3.0 | 0.6- 25.2 | [5, 6, 21, 23, 25, 26] | ||||||
| Death related to Cardiac Event | 12.3 | 8.3 | 3.2 | 0.2- 27.0 | [4-6, 18, 22, 28, 35] | ||||||
| Common Variables | |||||||||||
| Infection after hematoma | 11.9 | 11.9 | [12, 13] | ||||||||
| Death related to Infection | 4.7 | N/A | [16] | ||||||||
| Death unrelated to Infection and Cardiac Complications | 0.2 | N/A | [45] | ||||||||
Hematoma Risk
There is paucity of clinical data on hematoma risk in patients who undergo TKA or TKA related surgery in the first 30 days after CAS. One study suggested [17] significant major bleeding occurred in up to 31.0% of patients who underwent non-cardiac procedures (such as TKA) in the first 30 days after CAS while having discontinued their APT medication. The averaged published risk of hematoma formation was found to be at 9.3% between 1 and 6 months [17, 18] and in 2.4% between 6 months and 1 year [19] in all other groups that discontinued APT.
Continuation of APT resulted in a probability of moderate to severe bleeding in up to 43.8% but at averaged at 26.1% in the first 30 days [20-23] after CAS, 21.0% in the time lag of 1- 6 months [24] and 6.0% of between 6 months and a year [25].
Risk of Cardiac Complications
Patients that discontinued APT prior to TKA or TKA-related surgery the risk of cardiac complications was 23.2% in the first 30 days post CAS placement [17, 20, 21, 26, 27], 9.5% if TKA was done between 1 and 6 months post CAS placement [17, 28], and 7.0% if TKA was performed between 6 months and 1 year post CAS placement [28, 29].
The risk of a patient with a history of a CAS suffering a cardiac complications in the postoperative period of a TKA while being on APT was 7.4% during the first 30 days after CAS placement [5, 6, 23, 30], 3.2% between 1 and 6 months [5, 21, 30] and 3.0% between 6 months and 1 year [5, 6, 30].
Sensitivity Analysis
Some of the published data used for the computed model may not correspond to clinical experience. Therefore, to determine the rates of complications and account for the variations in the treatment strategies that would influence the decision to continue or to discontinue APT in those patients that undergo TKA or TKA related surgery, we conducted a threshold analysis [31]. The threshold is defined as the lowest probability at which the decision in favor of terminating antiplatelet regimen yields a lower expected mortality rate than the decision against. A low threshold implies a strong indication for a particular therapy.
We conducted sensitivity analysis to evaluate the effect of each variable on the outcome of the model. If a change of one variable alters the treatment strategy, the model is considered “sensitive” to that variable; if not, the model is deemed “robust”.
RESULTS
Mortality
The 30- day mortality after TKA or urgent TKA related surgery in patients with a history of recent (0-30 days) CAS placement and who discontinued APT prior to TKA, was calculated at 10.7% compared to an estimated 1.2% when APT was continued perioperatively. In patients that had a history of CAS between 1 month and 6 months prior to TKA, the 30- day mortality dropped to 2.6% in the group that discontinued APT versus 0.5% in those that continued APT. The 30- day mortality risk further dropped for patients undergoing TKA who had CAS between 6 months to 1 year prior to TKA to 1.6% (discontinued APT) and 0.3% among those who continued APT. (Fig. 3).
Sensitivity Analyses
30-Day Mortality Risk Related to Cardiac Complication
In order to achieve an equal 30- day mortality risk in both treatment strategies (continuation and discontinuation of APT) for patients who had CAS placement 0- 30 days prior to TKA, cardiac complications needed to drop from an average reported risk of 23.2% in the group who discontinued APT to 2.2% -- an approximate ten-fold decrease.
For those with a history of CAS placement between 1 month and 6 months from TKA, the necessary threshold was calculated to be at 1.4%, a seven-fold drop from the original 9.5% risk of cardiac complications in those with discontinued APT.
The necessary threshold for cardiac complications in those patients, who underwent CAS placement between 6 months and 1 year from TKA, was 0.7% from an initial 7.0%.
30-Day Mortality Risk Related to Death from Cardiac Complications
Further threshold analysis demonstrated that if the risk of death related to cardiac complications with APT discontinuation decreased below 4.3%, 3.7% and 9.5% from the initial published risks of 44.9%, 24.9% and 19.8% for the three time intervals between CAS and TKA or TKA related surgery, discontinuation would lead to lower post-operative mortality compared to the continuation of APT (Table 2).
Beyond 1 year, there was a minimal difference in the 30-day mortality risk (not shown) between both treatment strategies and small variations in the different variables could favor either regimen.
| Variables | 0-30 days | 1-6 mo | 6mo- 1 yr |
|---|---|---|---|
| Reduction necessary to reach threshold to favor Discontinuation of APT | 40.6% | 21.2% | 10.3% |
| Threshold to favor Discontinuation of APT | 4.3% | 3.7% | 9.5% |
30-Day Mortality Risk Related to Hematoma Formation
Patients who continued APT were reported to have a bleeding risk ranging from 9.5% to 43.8% in the three different time intervals after CAS. Although this is a wide range and a very high percentage rate, it did not substantially affect the 30-day mortality in any of the time intervals as the risk of cardiac complications had a stronger affect on mortality.
DISCUSSION
We reported on the 30-day mortality risk of two different treatment strategies involving the perioperative discontinuation or continuation of APT in patients with a history of a CAS placement before TKA or urgent TKA related surgery such as infection or periprosthetic fracture. We used published data for the computed model and applied a wide sensitivity analysis to account for variations.
We found that the 30-day mortality risk in patients with a history of CAS placement who discontinued APT prior to TKA or urgent TKA related surgery was higher compared to those patients who continued with APT for up to a year. In the first time interval of 0-30 days post CS placement, the 30-day mortality of TKA or TKA related surgery was calculated to be at 10.7% when APT was discontinued which was driven by the death related to cardiac complications and dropped to 2.6% and 1.6% in subsequent two time intervals (1- 6months and 6 months- 1 year). This is in contrast to 1.2%, 0.5% and 0.3% 30- day mortality risk in patients that continued APT, respectively.
There is a lack of consensus on the “safe” timing of orthopedic surgery after CAS, on the decision of continuation or discontinuation of APT prior to the procedure and if APT is stopped, the number of days that APT should be discontinued [32]. With an aging population and associated obesity and diabetes, which are main risk factors for cardiovascular disease the number of percutaneous interventions are likely to increase. In fact, the number of percutaneous interventions tripled over the period between 1988 to 2001 from 80.3 per 100,000 [95%CI: 71.9-88.9] in 1988 to 244 per 100,000 [95%CI: 221.3-266.4, p<0.01] in 2001 in the United States [33]. Delaying orthopedic procedures for osteoarthritic conditions such as a TKA or urgent TKA related procedures due to the presumed increased risk from cardiac complications that may ensue from recent CAS could lead to worsening impact on the patient’s quality of life [33]. Therefore, it is often left to the orthopaedic surgeon and the patient’s PCP and/or cardiologist to balance risk and benefits of the two main treatment strategies of APT when surgery is necessary.
Previous studies reported variable outcomes, including complications, such as cardiac events that may lead to death of non-cardiac surgery after drug-eluting stents or bare metal stents [5, 6, 18, 20, 22-24, 27-29, 34-36]. Drug-eluting stents have been reported to be associated with a higher rate of early restenosis and it has therefore been suggested to have those patients complete a full year of APT. It has been recommended to delay elective non-cardiac surgery >14 days for balloon angioplasty, >30-45 days for bare metal stents and >365 for drug eluting stents [37].
Small-scale studies have underscored the importance of completing a full course of one year of APT in the setting of necessary non-cardiac surgery [6]; however these findings are yet to be confirmed by larger and preferably randomized series. Kaluza and colleagues [20] were among the first to report on adverse events after CAS placement in patients who subsequently underwent non-cardiac surgery. In their study, the authors retrospectively assessed 40 patients who underwent non-cardiac surgery after recent CAS and reported on seven myocardial infarctions, 11 severe bleeds and eight deaths. In contrast to our study, they were not able to control for continuation or discontinuation of APT.
We used the available data from the literature and created two distinct treatment arms that allowed us to identify the strategy that was associated with a lower mortality rate followed by a sensitivity analysis to account for the variations of the treatment strategies. Vicenzi et al. [4] prospectively analyzed 103 patients that underwent non-cardiac surgery within the first year of CAS placement. APT was either withdrawn 3 days prior to the surgery or was continued throughout the perioperative period. In almost half of the patients (n=46), complications occurred postoperatively, consisting of 42 who had a cardiac event and two who had bleeding. They found an increased risk of adverse events in the immediate period after CAS placement (<35 days) compared to CAS placement more than 90 days prior to non-cardiac surgery. Their results are comparable to our findings. Cardiac complications appear to be the main risk in the perioperative period following non- cardiac surgery in the patient with recent CAS.
Maintaining non-cardiac surgery patients on APT increases the risk of bleeding as shown by Wilson et al. [23] who documented the need for blood transfusions in 33% of patients who underwent non-cardiac surgery including vascular, orthopaedic, genitourinary and gastrointestinal procedures while on APT. However, no studies have found a significant impact on overall mortality.
Using data from multiple data sources, our analysis was able to confirm this finding. In the current study, bleeding risk in patients who were kept on APT ranged from 9.5% to 43.8%, which did not influence the overall 30- day mortality for up to a year. A study reported [17] that patients who discontinued APT preoperatively for up to 7 days demonstrated an initial high bleeding risk (31.0%) in the first two weeks, which may reflect that patients may be at higher risk of bleeding in the first 30 days regardless of continuing or discontinuing APT. This may not reflect clinical expertise but this finding did not offset our sensitivity analysis. The mortality risk related to infection after hematoma formation or moderate to severe bleeding is low and therefore had little impact on the 30-day mortality compared to the 30-day mortality caused by cardiac complications. However, we were unable to measure morbidity related to hematoma formation with a potential of infection after TKA surgery.
It is noteworthy that there are several definitions of hematoma formation. While some define hematoma formation as the need for re-operation for bleeding [38] others such as the American College for Cardiology and American Heart Association elect to use the term “moderate hematoma” as a surrogate for hematoma formation [39]. We elected to use the later to potentially overestimate the amount of hematoma formation on purpose. Hematoma formation in essentially all patients with a 50% infection risk would favor the perioperative discontinuation of Plavix at any given time interval.
Jacob et al. [40] performed a retrospective analysis on 142 patients undergoing arthroplasty while being either off or on Plavix perioperatively and determined 30- day readmission, reoperation, hematoma formation. 3 patients developed bleeding and one infectious complication in the group that discontinued Plavix. They were unable to state the diagnosis for which Plavix was prescribed nor was there sufficient data on when a coronary stent was placed and the time lag between the coronary stent and the arthroplasty. We used their data on hematoma formation and transfusion requirement for our sensitivity analysis and our results were not impacted by their findings. Nandi et al. [38] performed a retrospective study assessing the effect of Plavix in the perioperative setting. They included patients being on Plavix for a prior stroke and peripheral vascular disease as well as a prior coronary stent. The data presented did not disclose the time between stenting and arthroplasty. Interestingly, there was no statistical difference between hematoma formation in either group of patient’s undergoing arthroplasty, however they noted a higher incidence of reoperation for hematoma formation in revision arthroplasty while being on Plavix. Similarly, using their data did not impact our conclusions since the risk of hematoma formation has little impact on mortality.
While in clinical practice surgeons may consider other strategies related to discontinuation of Plavix or aspirin or both, currently there is no published literature reporting differential hematoma formation rates among alternative clinical strategies beyond those described in our analysis. Further, results of our extensive sensitivity analyses suggested that variability in rates of hematoma had no effect on ranking of the strategies.
The important finding of this study is that the risk of hematoma formation that may be due to continuation of Plavix in the first year after coronary stenting has a minimal effect on the mortality since discontinuing Plavix increases the risk of cardiac complications, which is associated with a high mortality rate. For example, when TKA is performed between 1 month and 6 months after coronary stenting, the hematoma formation would have to virtually affect every patient (99%) with a risk of hematoma formation of 50% to offset the discontinuation of Plavix and cause a higher mortality.
Due to the heterogeneity of the available literature, limitations included the inability to differentiate between the type, number, length and diameter of the stent. This is a potential bias as it has been shown, that longer, drug-eluting stents may result in earlier occlusion. Further, it is known that CAS after acute coronary syndrome are associated with a higher incident of adverse events [6].
Other limitations consisted of the fact that we could neither differentiate between types of non-cardiac surgery, nor the overall health status of the assumed cohort that might have influenced the model. It is likely that TKA has a lower complication rate than, for example, elective vascular surgery. Similarly, a lower NYHA status could influence the outcome of cardiac complications. We therefore conducted a wide set of sensitivity analysis to evaluate the robustness of the model. In all simulated cases, cardiac complications were required to fall significantly up to eight fold to achieve a lower 30-day mortality risk than in the group that continued APT for up to year. The published data were too sparse and heterogeneous to permit stratification by diabetes, renal failure, different location of coronary artery stenosis and stent types, low ejection fraction and prior surgeries that could potentially skew the results, except for stenosis of the coronary vessels requiring intervention. We do not have external data against which we can validate the performance of the model. In these circumstances, sensitivity analyses are valuable tools for investigating the range of outcomes that might be expected from plausible swings in key input parameters. These analyses are particularly useful in models such as ours in which most input parameters were derived from observational and retrospective studies in the absence of randomized controlled trials.
This is the first study to our knowledge that elucidates the 30- day mortality risk of three different time intervals in patients who underwent CAS placement and subsequent TKA or urgent TKA related surgery such as infection or periprosthetic fracture. Although there are several limitations to this study, sensitivity analysis revealed the robustness of the decision model.
CONCLUSION
In conclusion, our data suggest that delaying TKA for at least 1 year after percutaneous CAS placement leads to substantial reduction in 30-day post-TKA mortality. In almost all scenarios, terminating APT prior to TKA or urgent TKA related surgery in the first year after percutaneous CAS placement carries a higher 30-day mortality than continuing APT perioperatively. We were, however unable to determine morbidity. The continuation of perioperative APT did not appear to have an influential effect on the rate of increased hematoma formation and subsequent infection rate. Therefore, postponing an elective TKA may lead to a substantial reduction in post- TKA mortality. Whenever urgent TKA related surgery is required, the orthopaedic surgeon should weigh risks and benefits of death, major cardiac issues and the deleterious effects of hematoma with each other. The complexity of this decision for every patient should prompt close consultation with the primary care physician, cardiologist and/or cardiologic interventional physician to provide the best possible outcome for the patient.
LIST OF ABBREVIATIONS
| APT | = Antiplatelet therapy |
| CAS | = Coronary Artery Stenting |
| TKA | = Total knee arthroplasty |
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
NIH/NIAMS K24 AR057827 (Dr. Losina), RO1 AR064320, P60 AR 47782, and the Department of Orthopedic Surgery-Program for Research Incubation and Development, Brigham and Women’s Hospital.


