All published articles of this journal are available on ScienceDirect.
The Rule of Histology in the Diagnosis of Periprosthetic Infection: Specific Granulocyte Counting Methods and New Immunohistologic Staining Techniques may Increase the Diagnostic Value
Abstract
Objective:
The current study investigates the diagnostic accuracy of the criteria described for frozen sections and whether modern leukocyte specific staining techniques including leukocyte peroxidase and Naphtol-AS-D-chloroacetate-esterase will improve the accuracy of the intra-operative histology.
Method:
77 patients undergoing revision total hip and knee arthroplasty were included in this retrospective study. Patients were grouped into septic and aseptic based on intraoperative cultures. Tissue samples were analyzed utilizing the Mirra, Feldman, Lonner, Banit and Athanasou criteria.
Results:
An experienced pathologist had a high specificity (96%), but rather low sensitivity (57%) diagnosing infection. By using the Banit-, Mirra-, or Athanasou-criteria the sensitivity is increased to 0.90. The Feldman- and Lonner-criteria have a lower sensitivity (0.48 and 0.38), however, an increased specificity of 0.96 and 0.98, respectively. The Banit cut off has the highest accuracy (86%). MPOX and NACE staining increased the sensitivity and accuracy up to 100% and 92% respectively.
Conclusion:
Banit’s cut off is the most accurate histologic criteria to diagnose infection. Modern leukocyte specific staining techniques slightly improve the accuracy. The synovial fluid white blood cell count appears to be the most accurate intraoperative test.
INTRODUCTION
Deep implant infection is the most common reason for revision total knee arthroplasty (25.2%) and the third most common reason for revision total hip arthroplasty (14.8%) [1, 2].
Considering the difference in treatment (one stage versus two stage) correct pre- or intraoperative diagnosis of infection is of utmost importance.
A combination of patient history, physical exam, laboratory work up and joint aspiration are often sufficient to diagnose deep implant infection [3]. Patients with increased BMI, diabetes mellitus, hypertension, steroid therapy and rheumatoid arthritis are at increased risk for infection [4]. Several studies showed that the erythrocyte sedimentation rate and C-reactive protein level are sensitive and specific for the diagnosis of infection in hip and knee arthroplasty [5-8]. The preoperative determination of the synovial fluid white blood cell count (WBC) is also an accurate test [6]. In addition some studies showed promising results using newer markers, such as Interleukin 6 (IL-6) [9, 10].
None of the currently available preoperative tests has the ability to predict the absence of a joint infection safely [6, 11, 12, 13]. In cases with an unclear preoperative workup the surgery is the last resource to verify the diagnosis. Unfortunately, although the intraoperative picture of deep implant infection seems to be characteristic, intraoperative evaluation by the surgeon has a rather low sensitivity (0.70), specificity (0.87) and accuracy (0.82) [14]. While new intraoperative tests like alpha defensin have been introduced, they lack behind the diagnostic accuracy of the laboratory alpha defensing tests and seem to be equivalent to frozen section [11, 15-19, 20-22]. Because of its universal availability intraoperative frozen section is often the last resort for the surgeon to rule out infection at the time of surgery.
Diagnostic criteria for frozen section have been described in the literature [14, 15, 23, 24, 25]. These criteria are based on the number of polymorphonuclear leukocytes (PML) per high power field in synovial tissue samples collected from the close vicinity of the revised implant and the interface membrane [14]. Diagnostic criteria range from at least one PML in 10 high power fields to at least one high power field with at least 10 PMLs [15, 24].
The current study investigates the diagnostic accuracy of the criteria established by Mirra et al. [23], Feldman et al. [14], Lonner et al. [25], Athanasou et al. [24], and Banit et al. [15] compared to the evaluation by an experienced pathologist and the synovial fluid WBC count. The study also analyses whether modern leukocyte specific staining techniques including leukocyte peroxidase (Myeloperoxidase: MPOX) and Naphtol-AS-D-chloroacetate-esterase (NACE) will improve the accuracy of the intra-operative histology.
METHODS
77 patients undergoing revision total hip and knee arthroplasty between August 2003 and March 2004 were included in this retrospective study. Patients were grouped into septic and aseptic revisions based on the intraoperative culture. During the surgery, two tissue samples were taken from the synovial tissue and the interface membrane. All patients were off antibiotic treatment for at least 2 weeks prior to surgery. Samples were sent for routine aerobic and anaerobic bacterial cultures. Tissue samples were cultured for 24-48 hours (standard culture) and 10 days (long term culture).
Based on the intraoperative culture, 56 patients were considered as aseptic revision while 21 patients were considered infected. Bacterial Pathogens were isolated form two separate tissue and fluid samples in all 21 patients and therefore all patients met the Musculoskeletal Infection Society (MSIS) criteria for deep implant infection [26]. Out of these 21 patients seventeen patients had preoperative joint aspirations with more than 20,000 white blood cells per milliliter and the remaining four patients had a fistula.
The aseptic group consisted of 28 male and 28 female patients with an average age of 66.6 years (range: 33.3 to 90.8 years). The septic group included 11 male and 10 female patients with an average age of 65.0 years (range: 20.0 to 84.8 years). During the surgery two tissue samples were taken from the inflamed pseudocapsule and the interface membrane in the technique described by Feldman et al. [14]. Care was taken to select pink-tan tissue samples and to avoid white scar tissue. Two specific samples were used to minimize the risk of sampling errors. Tissue samples were examined and sectioned at macroscopic examination, processed with a standard cycle, embedded in paraffin, cut at 5 microns, and stained with hematoxylin-eosin. In addition the same samples were stained using MPOX and NACE staining. The MPOX staining is performed using Anti-human myeloperoxidase (Code No. A 0398 Lot 096, DAKO A/S, Glostrub, Denmark) (Fig. 1). The NACE staining was performed using Naphtol-AS-D-Chloroacetat-esterase (Fig. 2). All samples were examined using the BX51 microscope (Olympus Deutschland GmbH, Hamburg, Germany). The most cellular areas were identified with a magnification power of four times. The number of polymorphonuclear leukocytes was counted using a magnification power of forty times. Infection was diagnosed using the criteria described by Mirra et al. Athanasou et al. Banit et al. Feldman et al. and Lonner et al. [14, 15, 23, 24, 25].
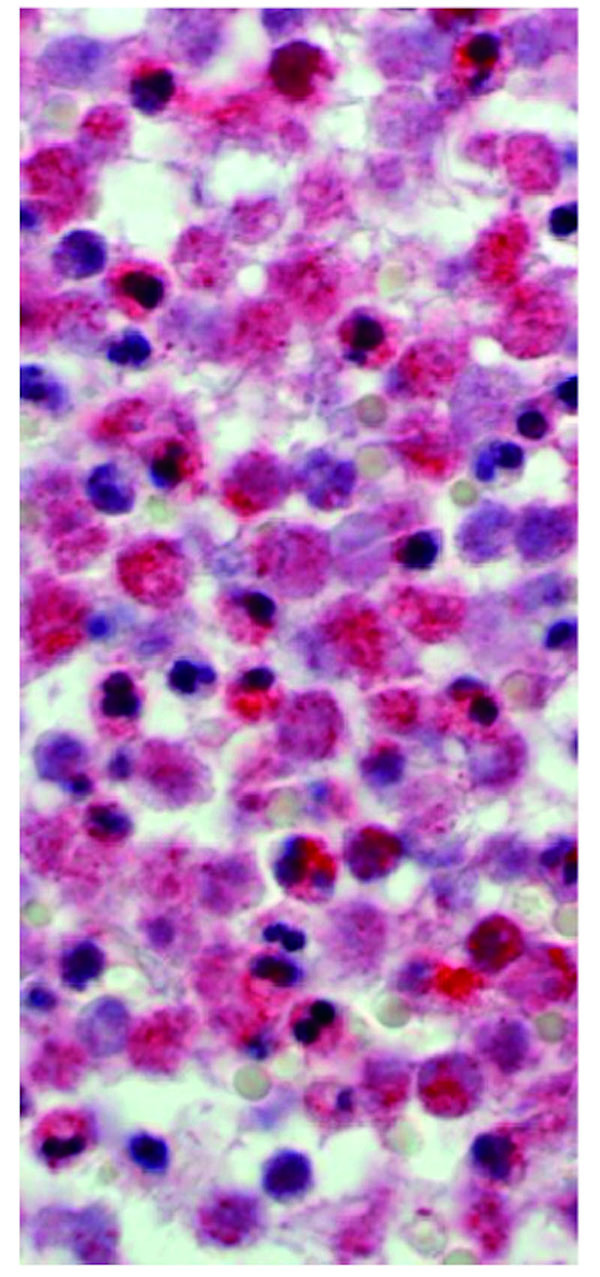
Mirra and coworker first reported on the correlation between quantitative analysis of the number of polymorphonuclear leukocytes per high power field and the presence of deep implant infection in 34 revision total joint arthroplasties. Mirra considered the frozen section to be suggestive of infection when one out of the five most cellular tissue regions showed more than five polymorphonuclear leukocytes per high power field [23].
In a similar technique Feldman et al counted the number of polymorphonuclear leukocytes in five high power fields (magnification power of 40x) in the five most cellular tissue samples [14]. A sample was considered positive for infection if there were more than five polymorphonuclear leukocytes in all five high power fields.
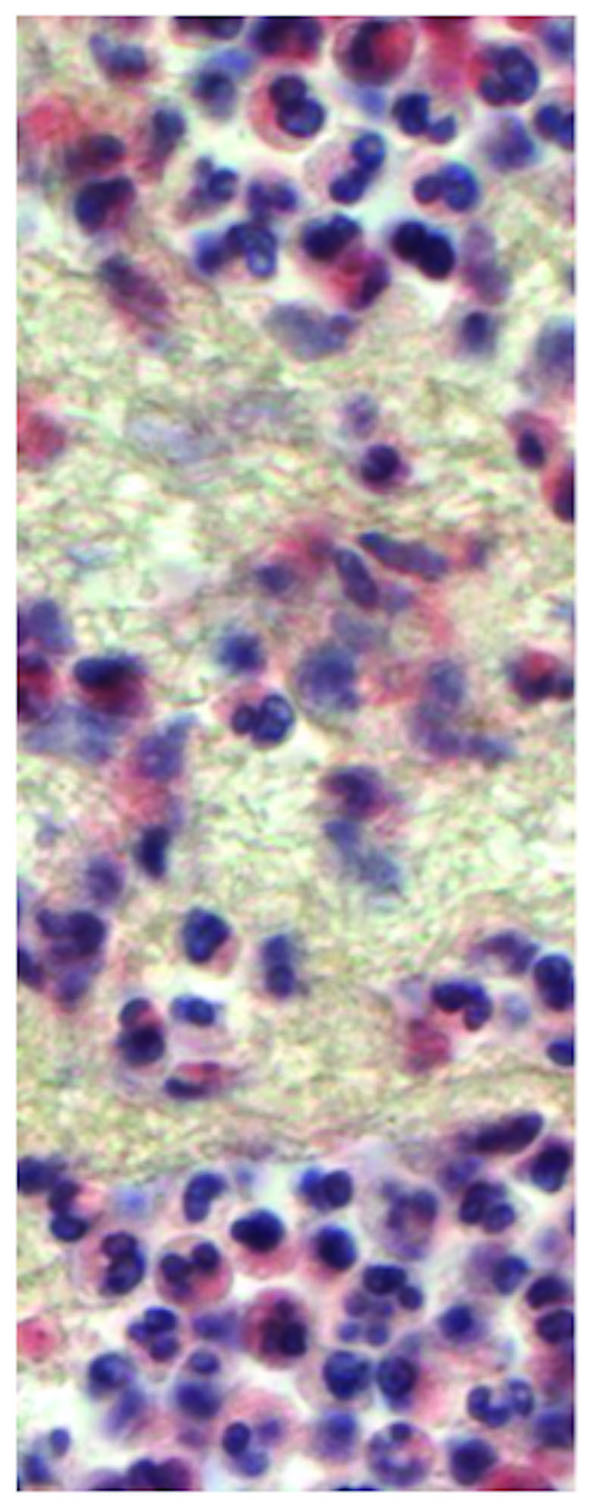
Lonner and coworker from the same institution published one year later that increasing the cut off to 10 polymorphonuclear leukocytes increased the specificity and accuracy of frozen sections [25].
Banit and coworker diagnosed infection if one out of the five most cellular high power fields showed more than 10 polymorphonuclear leukocytes [15].
Athanasou and coworker increased the number of examined high power fields to ten. In their study a deep implant infection was suspected if all ten high power fields showed at least one polymorphonuclear leukocyte [24].
An orthopedic pathologist with more than 14 years experience in the examination of periprosthetic tissue performed the general histologic evaluation and independently diagnosed infection based on the overall clinical picture, however, no set PML per high power field (HPF) cut off level was utilized at the time of this initial evaluation of the frozen section. Evaluations based on the described PML/HPF criteria and the immunohistologic staining were done at a later time point.
Based on the described criteria, sensitivity, specificity, positive predictive value, negative predictive value and accuracy were calculated using MedCalc Software (Medcalc, Mariakerke, Belgium).
Prior to perform the study approval was obtained from the Institutional Review Board at the authors’ institution.
RESULTS
An experienced pathologist falsely classified only 2 out of 56 tissue samples as infected (Specificity: 0.96), however, at the same time the pathologist only identified 12 out of 21 tissue samples with deep implant infection (Sensitivity 0.57) (Table 1). Requiring all five high power fields to show more than five polymorphonuclear leukocytes (Feldman et al 1995) or more than 10 polymorphonuclear leukocytes (Lonner et al 1996), decreases the sensitivity of the histologic analysis to 0.48 and 0.38 respectively (Table 1). Focusing the evaluation on the one high power field with the most polymorphonuclear leukocytes as recommended by Mirra et al. Banit et al. and Athanasou et al. increases the sensitivity to 0.90. Regardless of the evaluation criteria the accuracy of standard frozen section does not exceed 0.86 (Table 1).
| WBC count in synovial fluid | Pathologist | Feldman [14] | Lonner [25] | Mirra [23] | Banit [15] | Athanasou [24] | |
|---|---|---|---|---|---|---|---|
| Cut Off | > 12000 WBC / mL | Histologic picture | 5 HPF all showing more than 5 PML | 5 HPF all showing at least 10 PML | 5 HPF one showing at least 5 PML | 1 out of 5 HPFwith more than 10 PML | 10 HPF having an average of more than 1 PML |
| Sensitivity | 1.00 | 0,57 | 0.48 | 0.38 | 0.90 | 0.90 | 0.90 |
| Specificity | 1.00 | 0,96 | 0.96 | 0.98 | 0.71 | 0.84 | 0.67 |
| PPV | 1.00 | 0,86 | 0.83 | 0.89 | 0.54 | 0.70 | 0.51 |
| NPV | 1.00 | 0,86 | 0.83 | 0.81 | 0.95 | 0.95 | 0.95 |
| Accuracy | 1.00 | 0,86 | 0.83 | 0.82 | 0.76 | 0.86 | 0.73 |
The combination of different criteria to increase the accuracy of the test seems to have little effect (Table 3). To combine the different tests first all patients with infection are identified using the criteria with the highest sensitivity (Banit et al. 2002). In the next step all positive test samples are evaluated using a more specific criteria (Feldman et al. 1995). Using this technique the overall accuracy of the standard hematoxylin-eosin staining is 0.83 (Table 3). This is not more accurate than the Banit criteria alone.
Using leukocyte peroxidase (MPOX) to specifically stain polymorphonuclear leukocytes increases the sensitivity of all four different diagnostic criteria (Table 2). The criteria established by Mirra et al. Banit et al. and Athanasou identify all patients with deep implant infection (Sensitivity: 1.00). At the same time the specificity of these criteria decreases to 0.55, 0.73 and 0.50, respectively (Table 2). The specificity only increases for the criteria, which rely on evaluation of all five high power fields (Feldman et al. Lonner et al.). The combination of highly sensitive criteria (Banit et al. 2002) with very specific criteria (Feldman et al. 1995) as described above provides both high sensitivity and high specificity. If Banit’s criteria are used to screen for infection and Feldman’s criteria are used to further evaluate all positive tests, than the specificity can be increased to 1.00 while the sensitivity is: 0.76. This combination of the two criteria provides the highest overall accuracy in the current study: 0.94 (Table 3).
Naphtol-AS-D-chloroacetate-esterase (NACE) staining increased the sensitivity of all four diagnostic criteria compared to standard hematoxylin and eosin staining (Table 2). While the criteria established by Mirra et al, Banit et al and Athanasou et al showed a high sensitivity (Sensitivity: 0.95) its specificity was decreased to 0.54, 0.68 and 0.52 similar to the MPOX-staining (Table 2). Also similar to the MPOX staining the specificity increased for Feldman’s and Lonner’s criteria to 1.0. These criteria showed the highest overall accuracy of any single criteria test in the study (0.91 and 0.90). In contrast to MPOX staining the combination of different criteria did not improve the overall accuracy. The synovial fluid WBC count with a cut-off level of 12,000 WBC/mL was the most accurate test in the current study.
| Study | Feldman [14] | Lonner [25] | Mirra [23] | Banit [15] | Athanasou [24] | |
|---|---|---|---|---|---|---|
| Cut off | 5 HPF all showing more than 5 PML | 5 HPF all showing at least 10 PML | 5 HPF one showing at least 5 PML | 1 out of 5 HPFwith more than 10 PML | 10 HPF having an average of more than 1 PML | |
| HE-staining | Sensitivity | 0.48 | 0.38 | 0.90 | 0.90 | 0.90 |
| Specificity | 0.96 | 0.98 | 0.71 | 0.84 | 0.67 | |
| PPV | 0.83 | 0.89 | 0.54 | 0.70 | 0.51 | |
| NPV | 0.83 | 0.81 | 0.95 | 0.95 | 0.95 | |
| Accuracy | 0.83 | 0.82 | 0.76 | 0.86 | 0.73 | |
| MPOX-staining | Sensitivity | 0.76 | 0.71 | 1.00 | 1.00 | 1.00 |
| Specificity | 0.98 | 1.00 | 0.55 | 0.73 | 0.50 | |
| PPV | 0.94 | 1.00 | 0.46 | 0.58 | 0.43 | |
| NPV | 0.92 | 0.90 | 1.00 | 1.00 | 1.00 | |
| Accuracy | 0.92 | 0.92 | 0.68 | 0.81 | 0.64 | |
| NACE-staining | Sensitivity | 0.67 | 0.62 | 0.95 | 0.95 | 0.95 |
| Specificity | 1.00 | 1.00 | 0.54 | 0.68 | 0.52 | |
| PPV | 1.00 | 1.00 | 0.43 | 0.53 | 0.43 | |
| NPV | 0.89 | 0.87 | 0.97 | 0.97 | 0.97 | |
| Accuracy | 0.91 | 0.90 | 0.65 | 0.75 | 0.64 | |
| Study | Banit [15] | Feldman [14] | |
|---|---|---|---|
| Cut off | 1 out of 5 HPFwith more than 10 PML | 5 HPF all showing more than 5 PML | |
| 1st step | 2nd step (only positive results) | ||
| HE-staining | Sensitivity | 0.48 | |
| Specificity | 0.96 | ||
| PPV | 0.83 | ||
| NPV | 0.83 | ||
| Accuracy | 0.83 | ||
| MPOX-staining | Sensitivity | 0.76 | |
| Specificity | 1.00 | ||
| PPV | 1.00 | ||
| NPV | 0.92 | ||
| Accuracy | 0.94 | ||
| NACE-staining | Sensitivity | 0.67 | |
| Specificity | 1.00 | ||
| PPV | 1.00 | ||
| NPV | 0.89 | ||
| Accuracy | 0.91 | ||
DISCUSSION
The treatments of aseptic and septic implant failures differ fundamentally. Correct identification of patients with deep implant infection is therefore of outmost importance in revision total joint replacement.
Our data suggest that Banit’s criteria and the evaluation by an experienced pathologist are the most accurate histologic criteria to diagnose infection. Modern leukocyte specific staining techniques did only slightly improve the accuracy of the histologic examination if a single diagnostic criteria is used. An increased intraoperative synovial fluid WBC (>12,000 WBC/mL) was the most accurate test in the current study.
In 1973 Charosky et al. reported on the importance of the intraoperative histology for the assessment of periprosthetic joint infections. The tissue was stained with HE and classified as acute or chronic based on the predominant cell type. While the present of polymorphonuclear leukocytes was classified as an acute infection, lymphocytes and plasma cells were more suggestive of a chronic infection.
Mirra et al. pursued this concept and introduced the counting of PML per high power fields in 5 cell-rich areas. Feldman et al. took up this idea in 1995 and showed that these criteria are accurate for frozen section analysis and subsequent histological examination (correlation 100%). Nine intraoperative cultures were positive and all of the corresponding frozen sections showed more than 5 leukocytes per high power field. This resulted in a sensitivity of 1.00. In addition, of the 24 patients having negative cultures, only one patient had a positive frozen section (specificity 0.96). In comparison to criteria from other study-groups (e.g. Banit et al.) Feldman et al. demanded more than five polymorphonuclear leukocytes in at least five distinct high power fields.
The current study does not support the use of Feldman’s criteria for standard hematoxylin-eosin staining, since frequently at least one high power field showed equal or less than 5 PML. Therefore the sensitivity of the Feldman criteria was low (0.48). However, if the test criteria are fulfilled there is almost no doubt about the presence of an infection (specificity 0.96). The alternative staining techniques confirm the high specificity of the Feldman criteria. In our opinion the Feldman criteria should not be used as a screening test. This weakness was reported by Athanasou et al. in a letter to the editor [27]. Athanasou et al. suggested as an alternative concept to diagnose a deep infection in presence of at least one PML in 10 high power fields [24, 27].
All the above-described methods need a counting of the PML per high power field. Polymorphonuclear leukocytes (PML) play a decisive role in the defense of bacterial infections. They phagocytize bacteria and store them in phagosomes. The content of the cytoplasmic granules is released into the phagosomes. The granules contain various enzymes that destroy and break down the bacteria.
Myeloperoxidase is one of these enzymes, adding to around 5% of the dry matter. Myeloperoxidase is an important marker for polymorphonuclear leukocytes [28]. In different studies antibodies against myeloperoxidase were used to determine the extent of the inflammation [29-31]. We marked and stained the myeloperoxidase with Rabbit Anti-Human-Myeloperoxidase. Our data (Table 2) show that this procedure seems to be beneficial, especially when the diagnosis criteria demand a minimal number of neutrophil granulocytes in all high power fields. This applies to the Feldman and Lonner criteria. For all criteria MPOX staining increased the sensitivity. Therefore patients that did not have a corresponding number of granulocytes with the MPOX staining, have the highest probability to be infection free. However, the accuracy of MPOX staining does only slightly exceed HE staining (Table 2).
The cytoplasma of immature and mature neutrophil granulocytes contains a Naphtol-AS-D-chloroacetate-esterase (NACE) [32, 33]. In the presence of this esterase, Naphtol-AS-D-chloroacetate splits off Naphtol. Naphtol can be stained in red with a diazonium salt. The NACE does not stain the lymphocytes, monocytes, plasma cells, megakaryocytes, red blood cells, eosinophil and basophil granulocytes since they do not have any esterase activity. Both leukocyte specific staining techniques increase the sensitivity but the accuracy is not improved for all criteria.
Although a recent review of 26 studies involving 3269 patients undergoing revision hip and knee arthroplasty verified the good predictive value of frozen section in the diagnosis of deep implant infection [20] the overall accuracy was low. Nevertheless the authors recommended the frozen section analysis to be a valuable part of the diagnostic work up for patients undergoing revision joint arthroplasty.
The current paper has the following limitations: (1) leukocyte specific staining techniques (MPOX and NACE) are more time consuming than frozen section since the samples must be embedded in paraffin. This takes more time, so consequently the results will not be available during the surgery. Therefore these staining techniques have no value for the intraoperative decision-making. (2) the pathologist’s evaluation was not based on frozen sections but was made blinded at a later time point based on paraffin embedded sections. In the literature the correlation between frozen section and paraffin embedded routine sections is between 97% and 98.5% [18, 34]. (3) Only one pathologist performed the evaluation in the current study, and no attempt was made to determine intra and inter observer reliability. (4) In addition the paper excludes patients with rheumatoid arthritis and periprosthetic fractures and its conclusions cannot be applied to these groups of patients. (5) Since no patients with a metal on metal bearings or evidence of corrosion were included in the current study the conclusion needs to be applied with caution to patients with adverse local soft tissue reactions secondary to metal on metal bearings or corrosion.
SUMMARY
Banit’s cut off is the most accurate intraoperative histologic criteria to diagnose infection. Modern leukocyte specific staining techniques slightly improve the accuracy. The synovial fluid white blood cell count appears to be the most accurate intraoperative test and the current paper supports its value for the MSIS diagnostic criteria of infection.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


